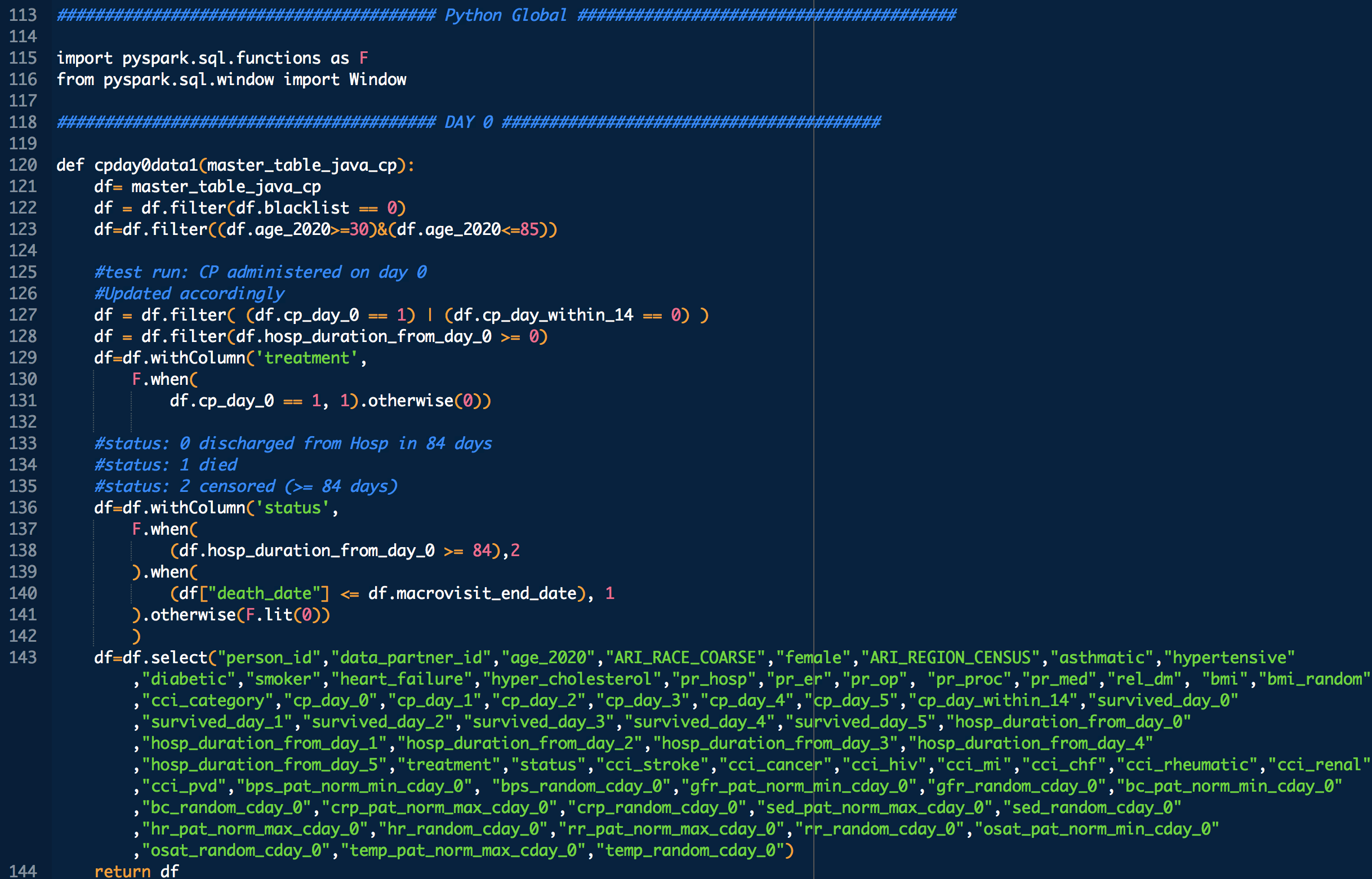
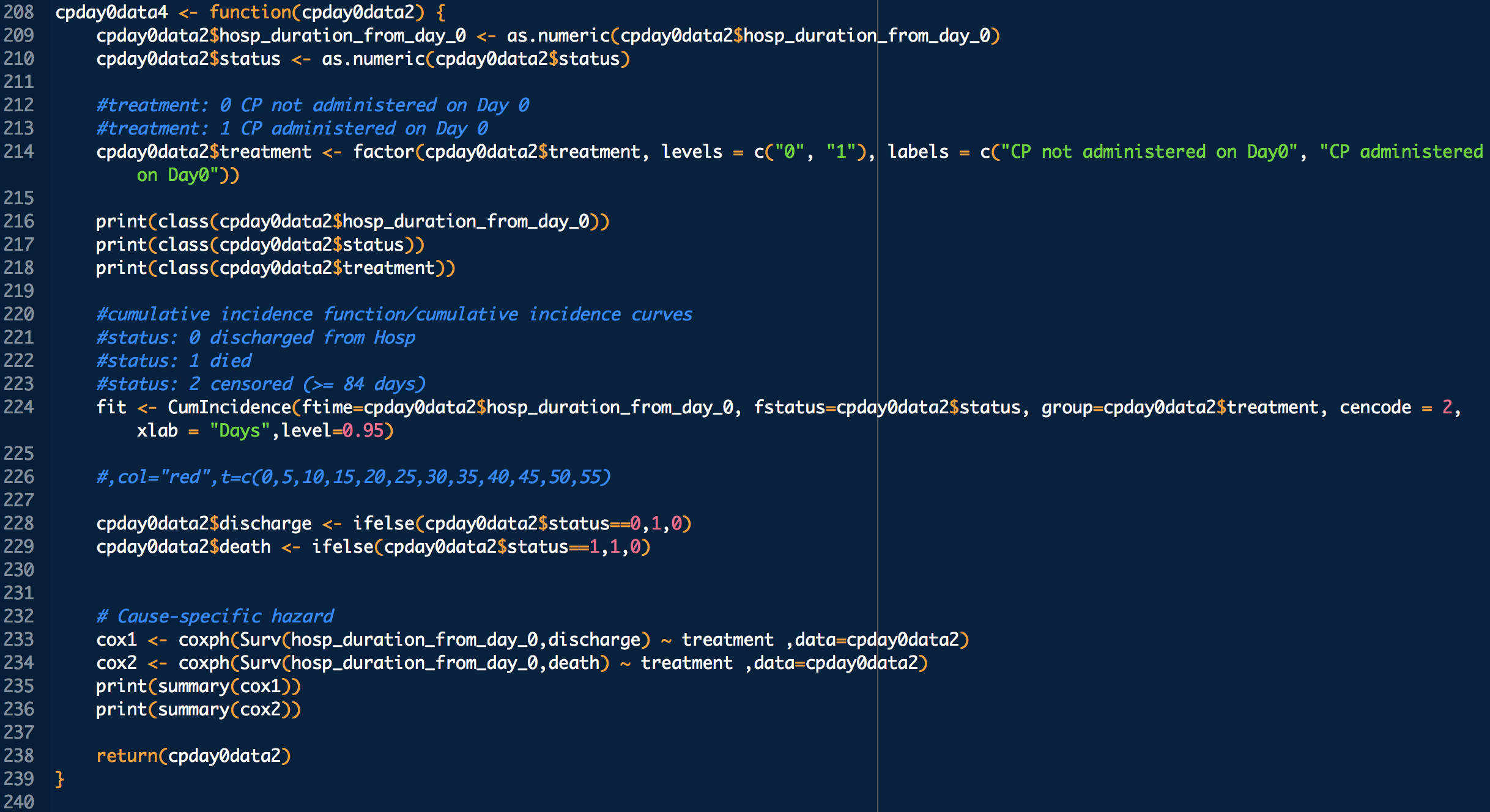
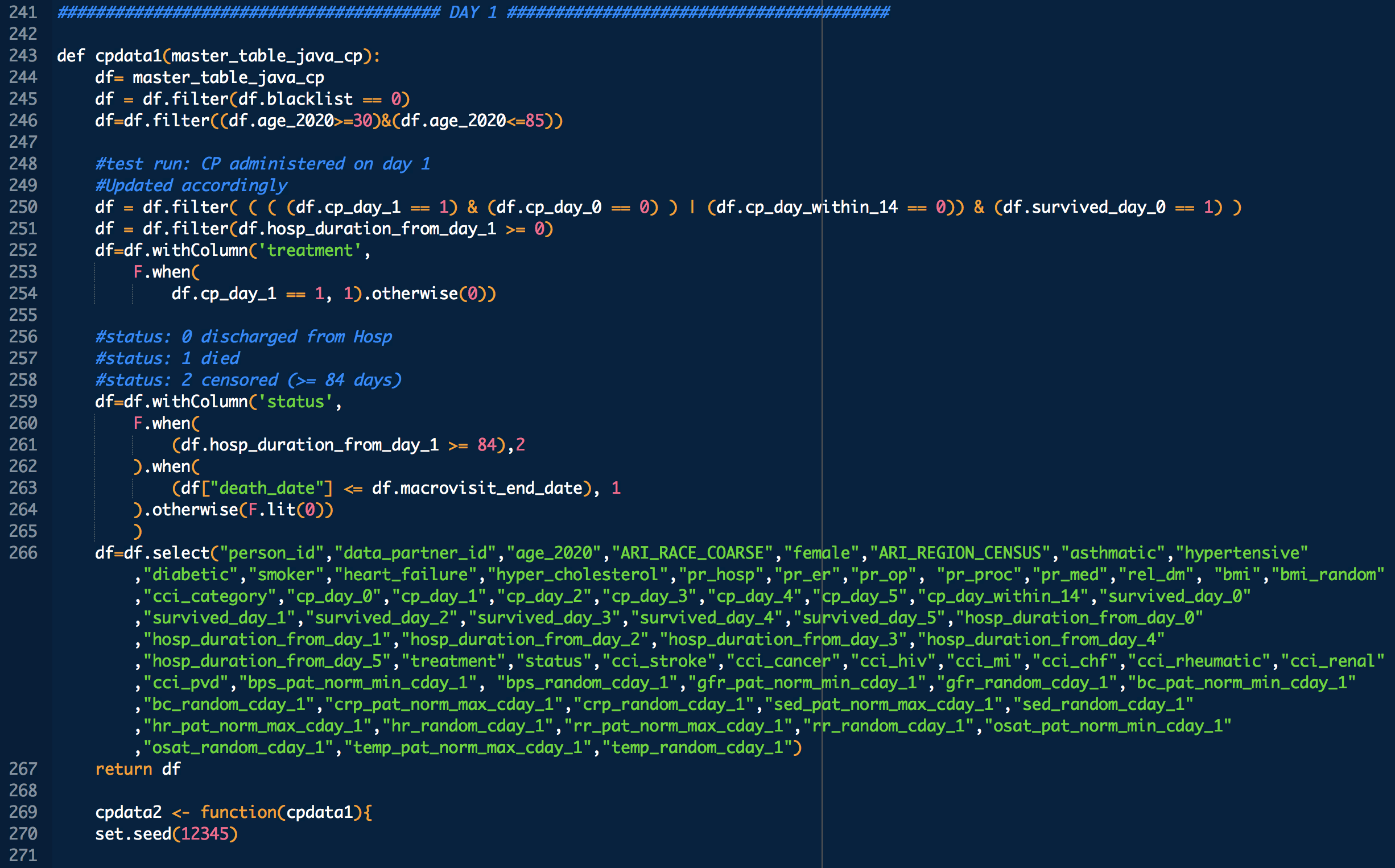
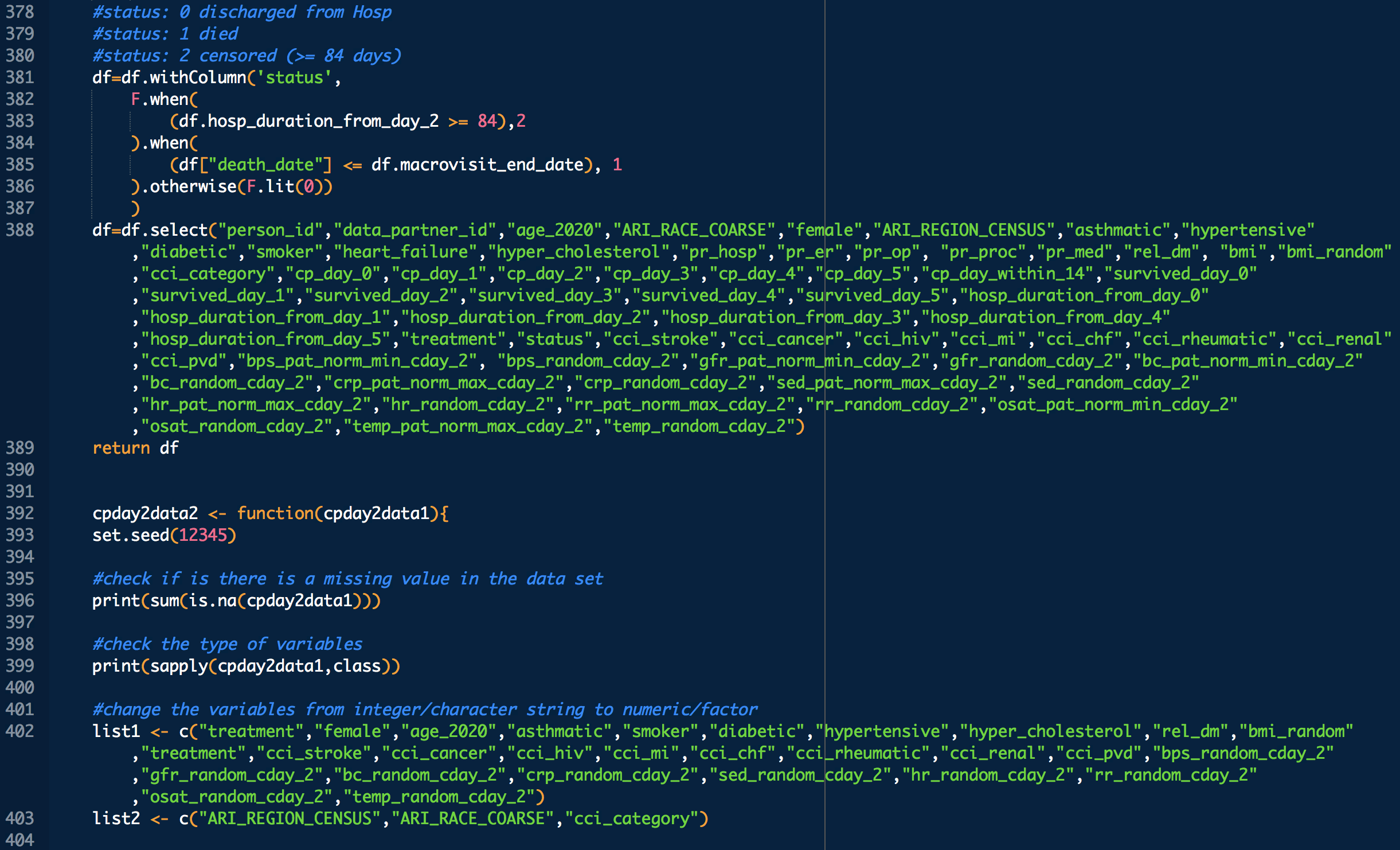
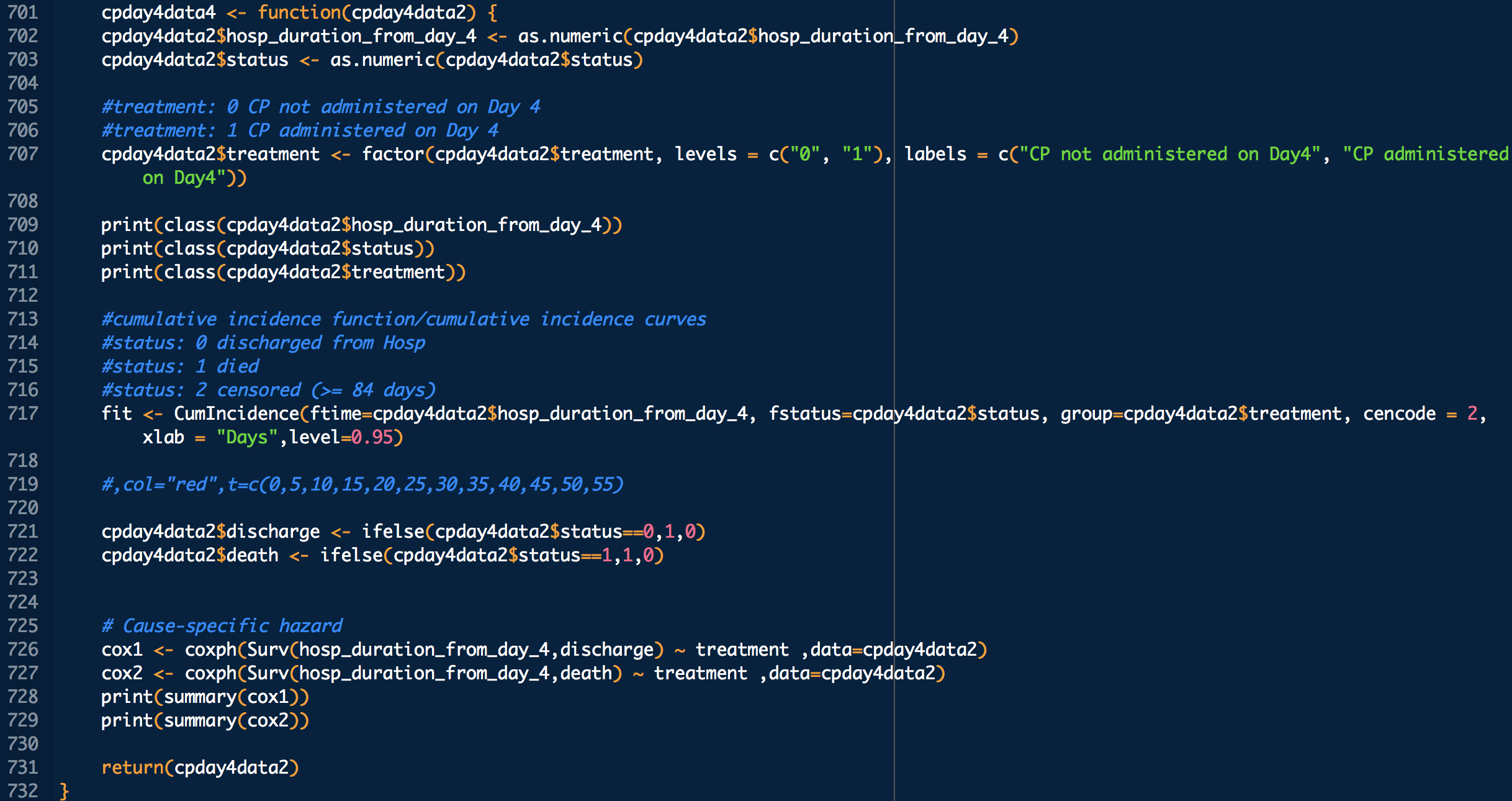
Administration day stratified analysis of convalescent plasma use shows no effect on COVID-19 hospitalization length
Applicable demographic/clinical distribution information based on 12-19-2020 N3C Data Release (Release v16). Supplementary materials of manuscript.
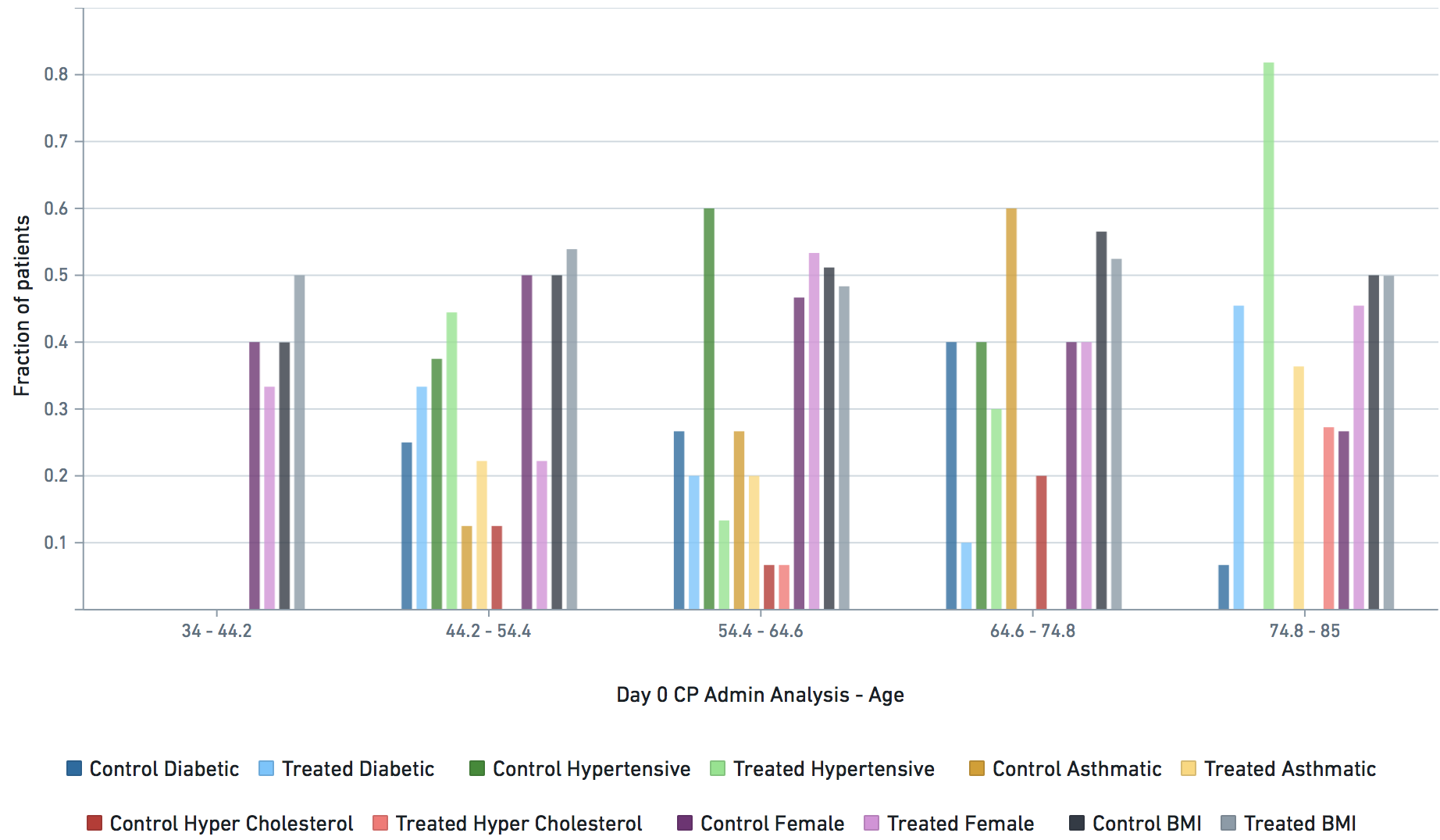
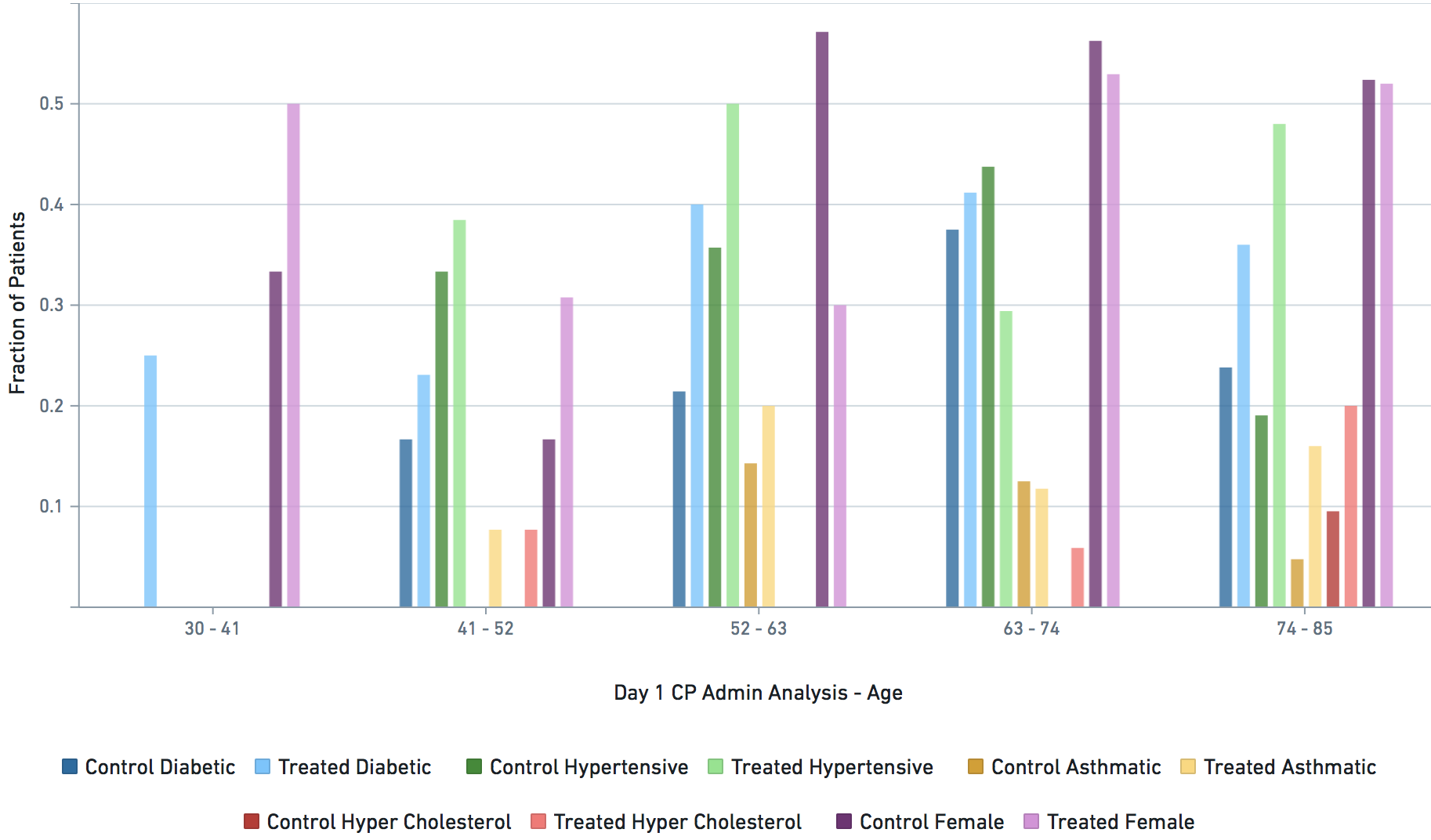
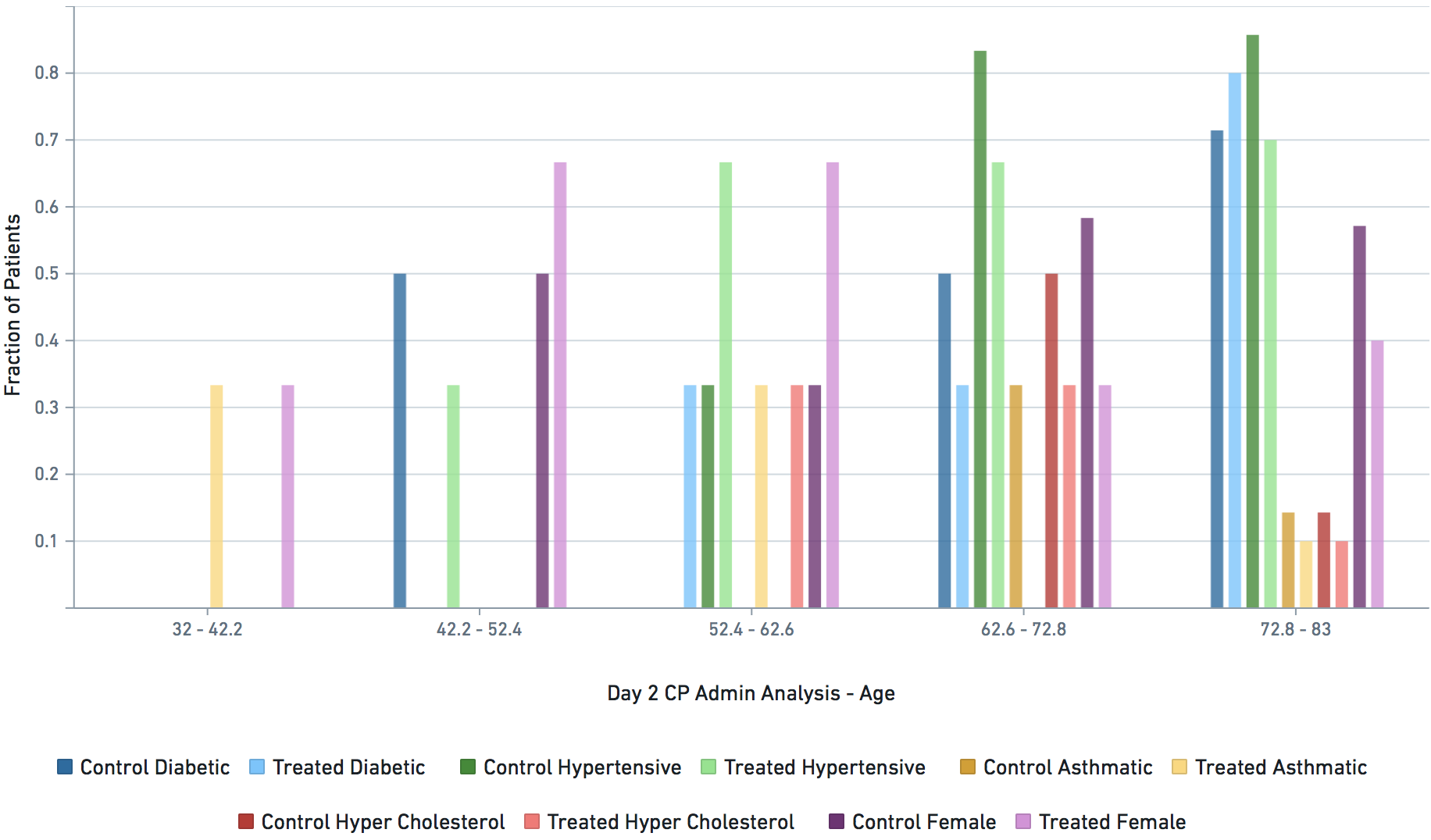
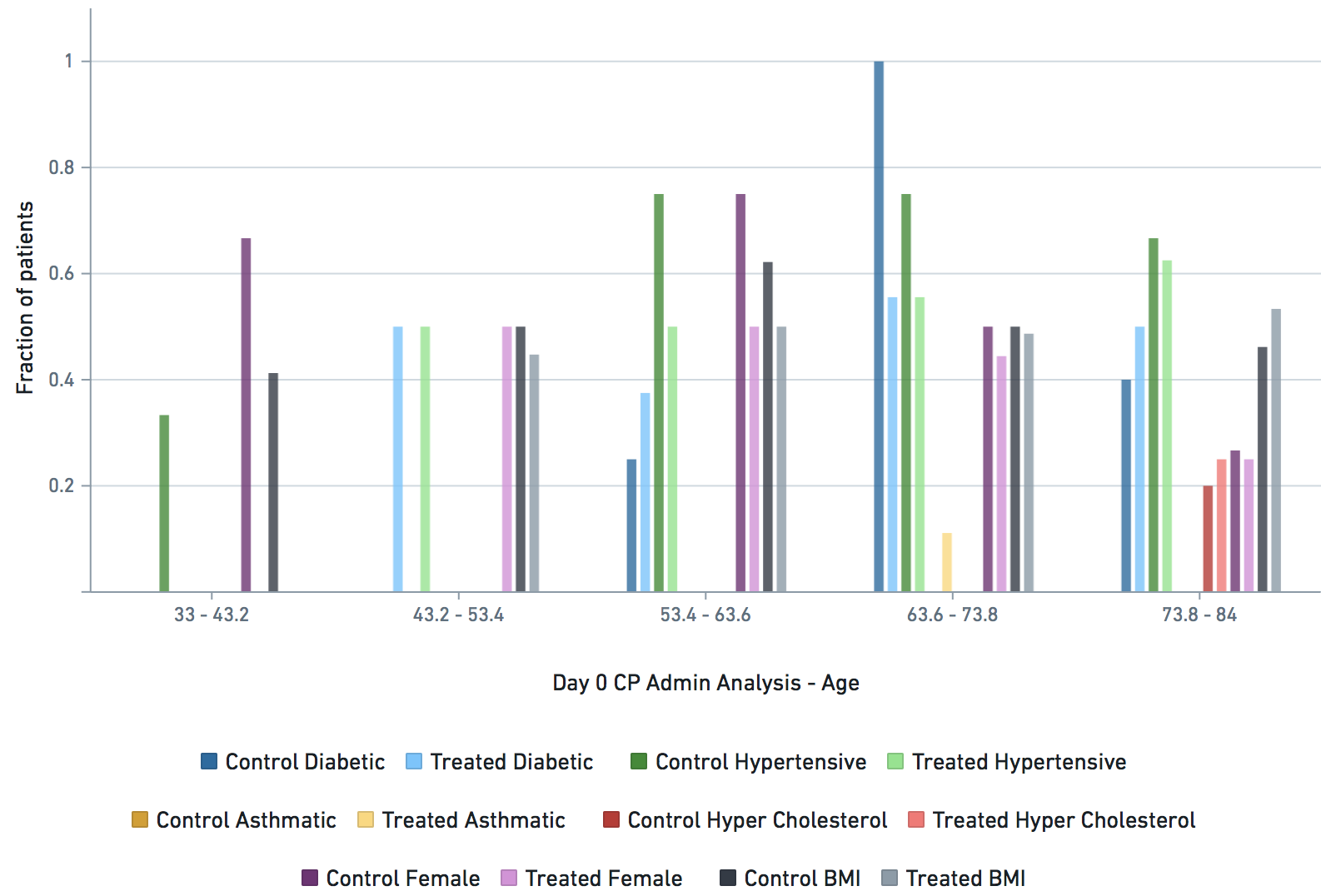
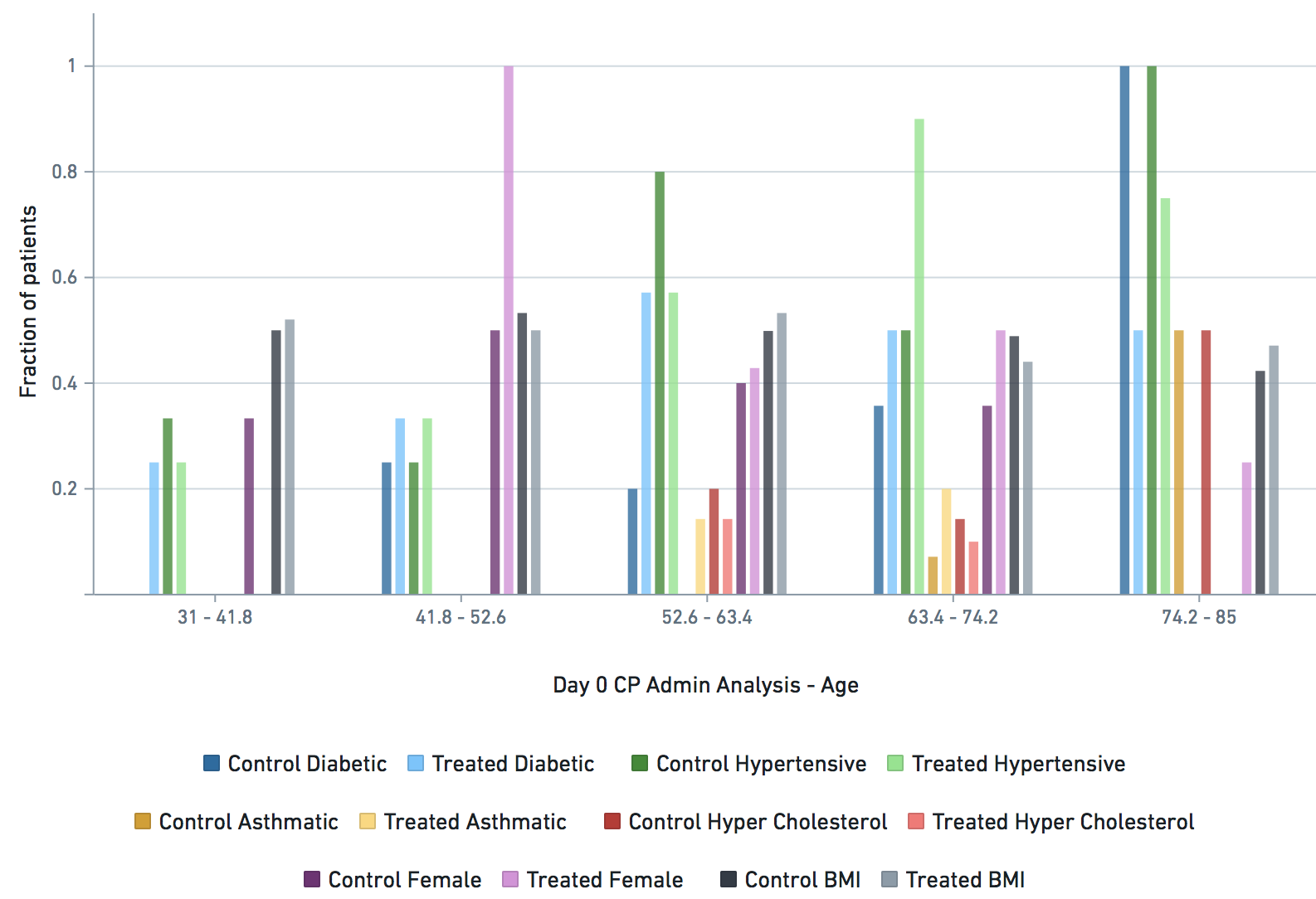
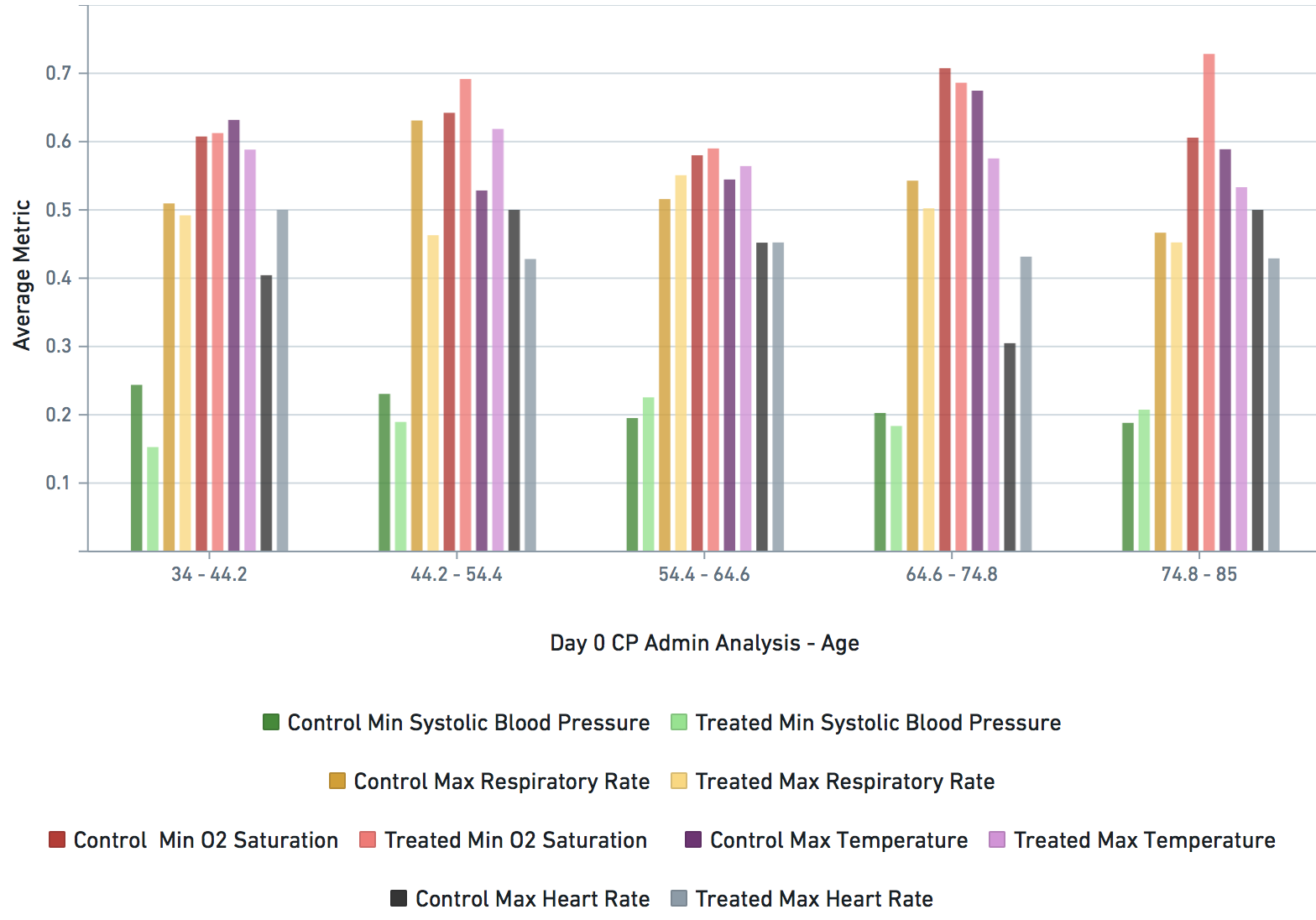
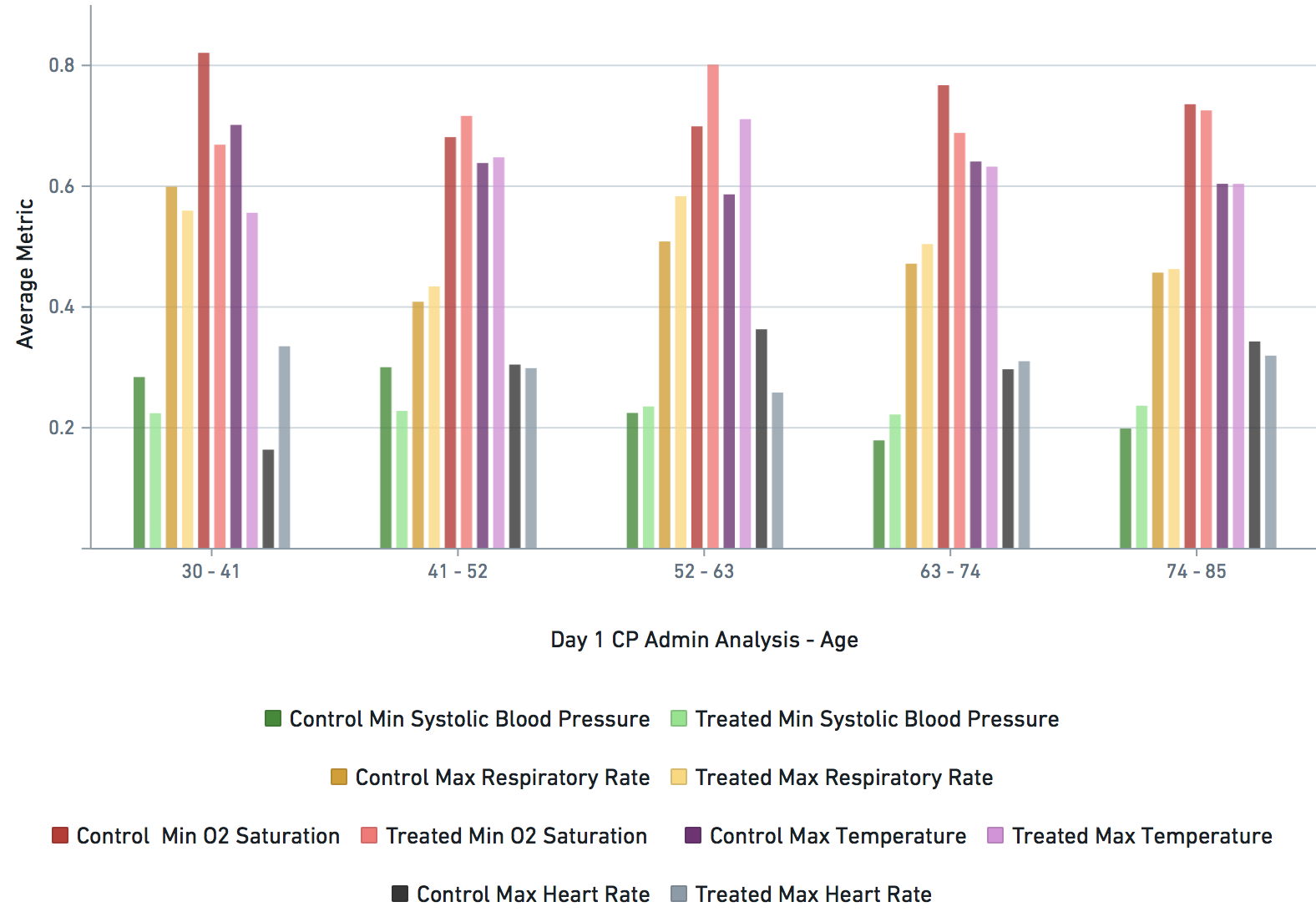
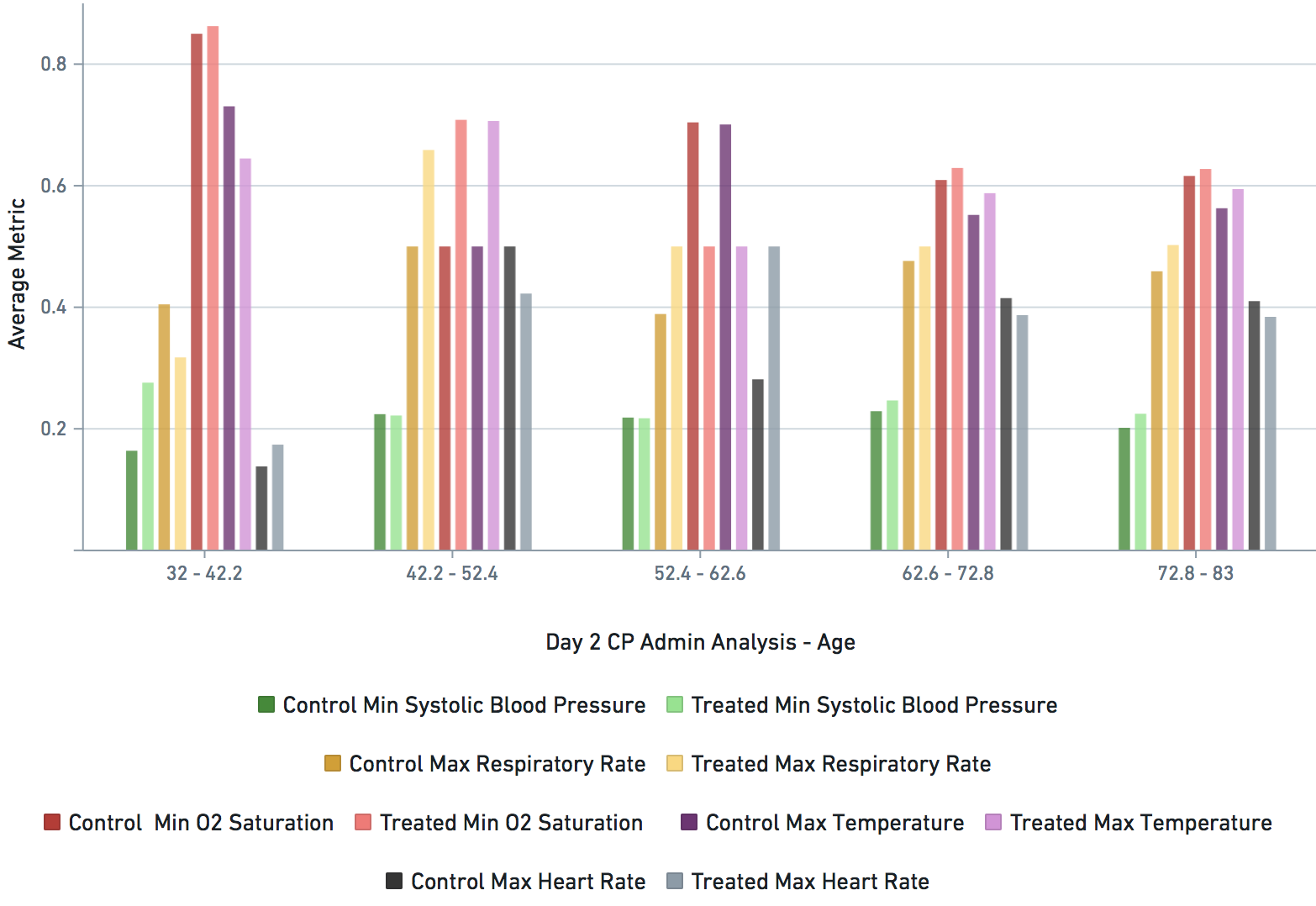
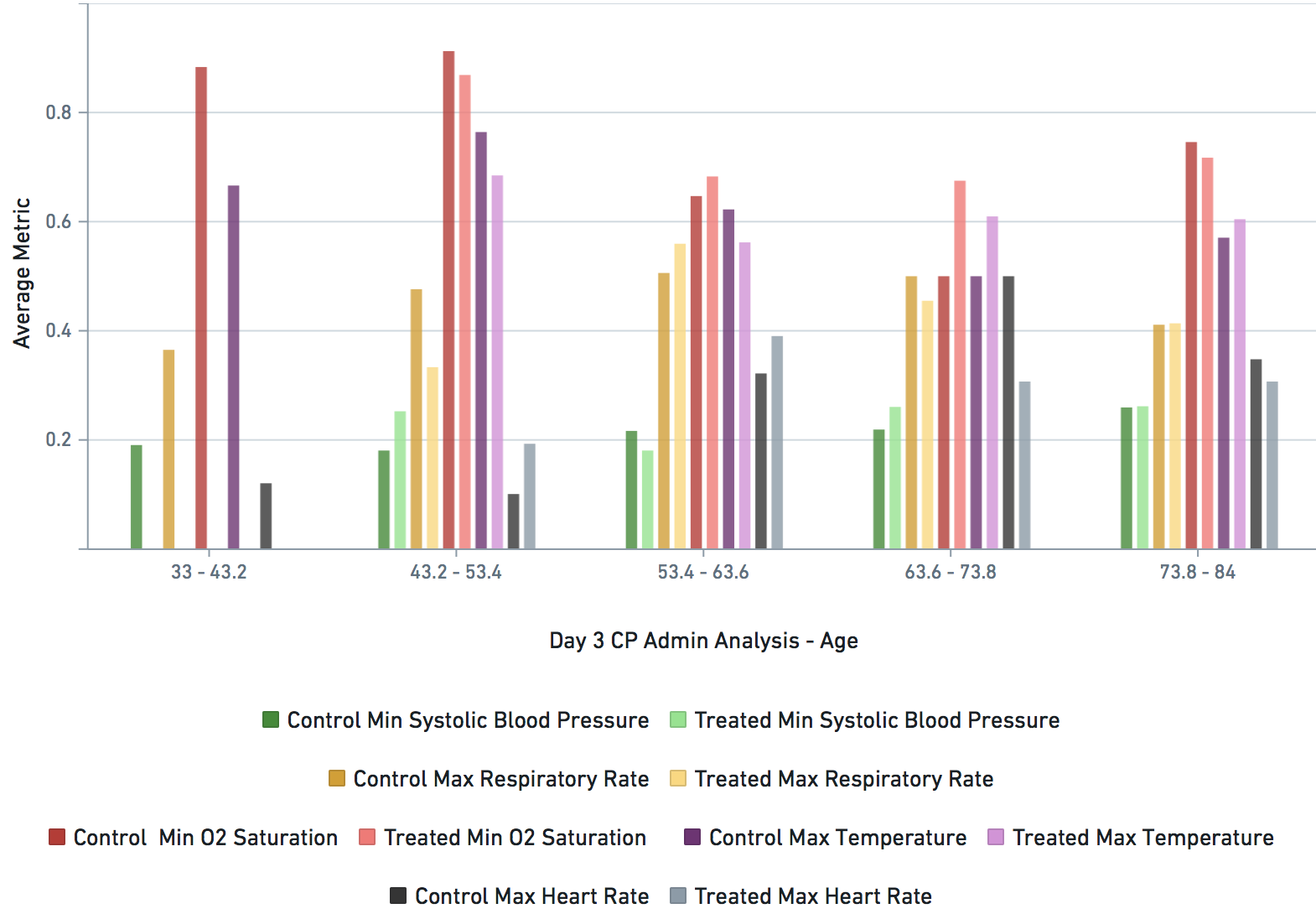
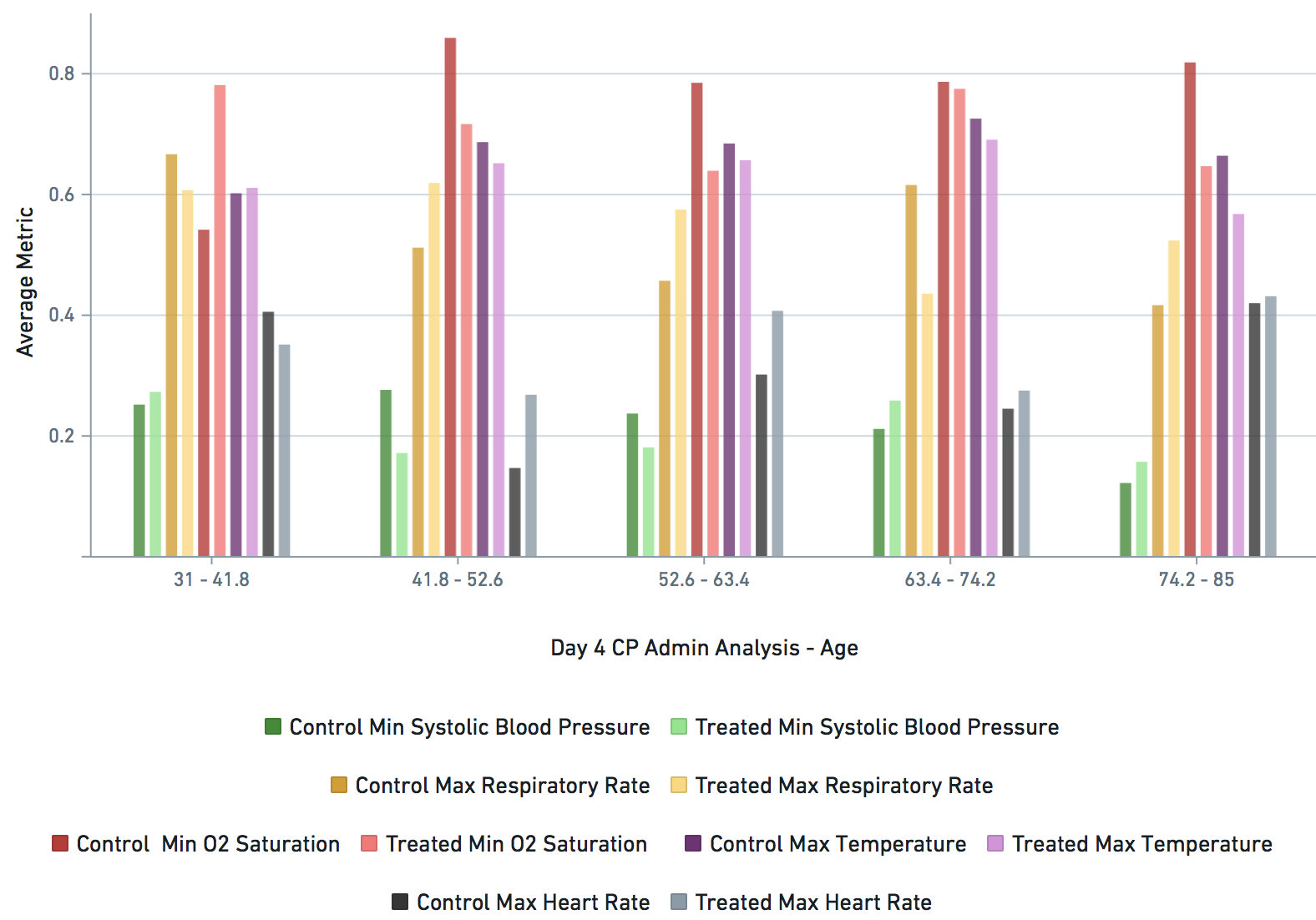
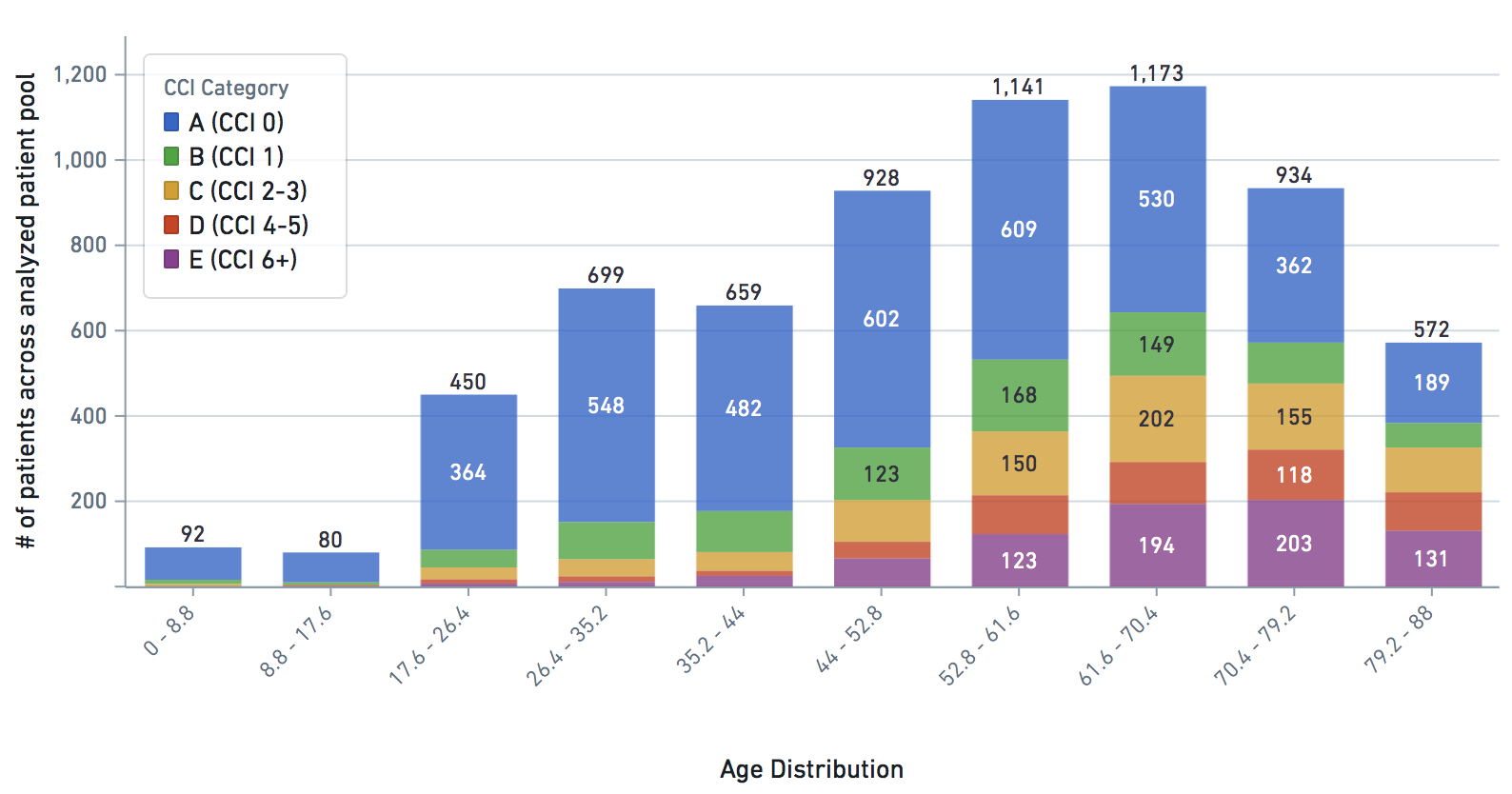
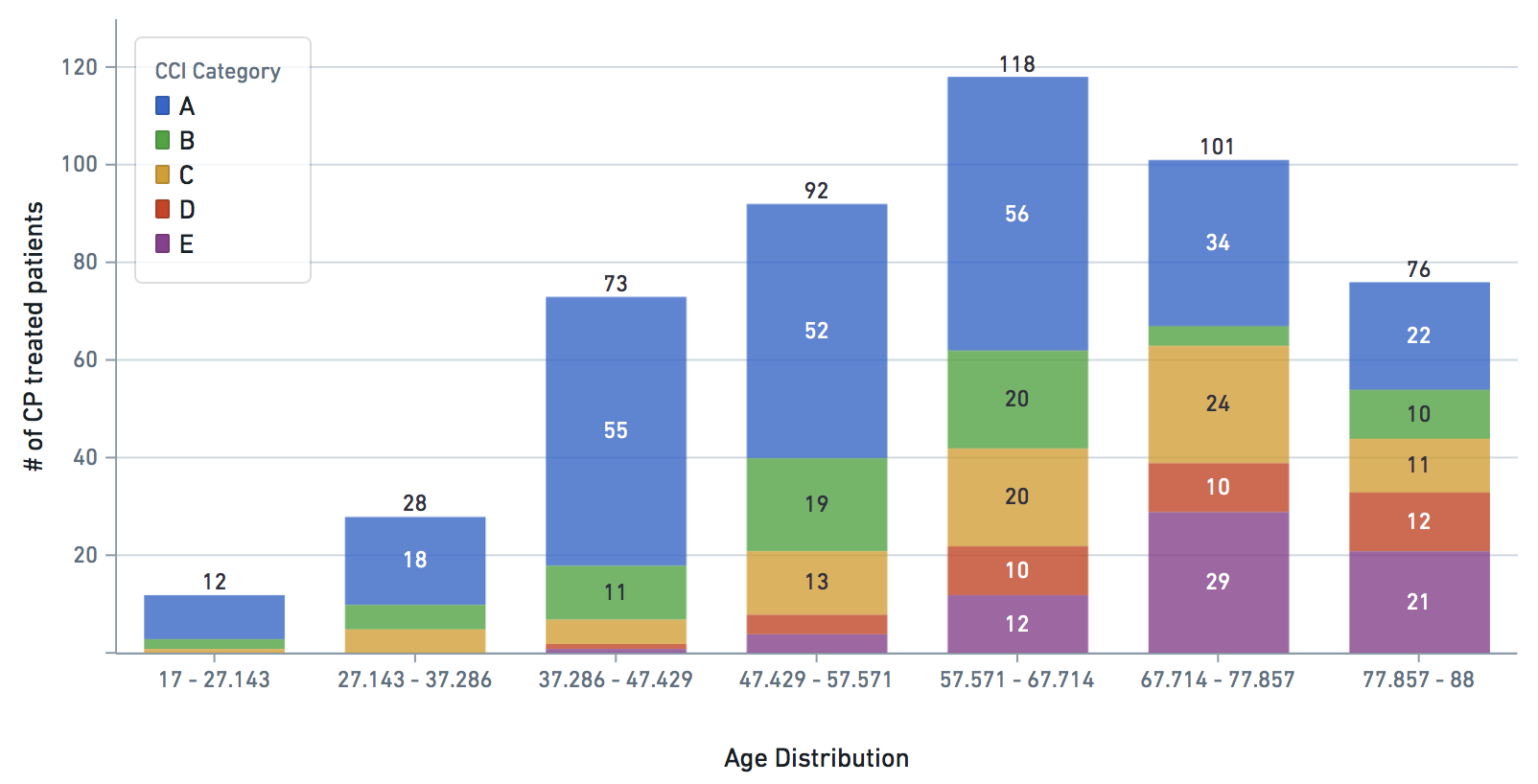
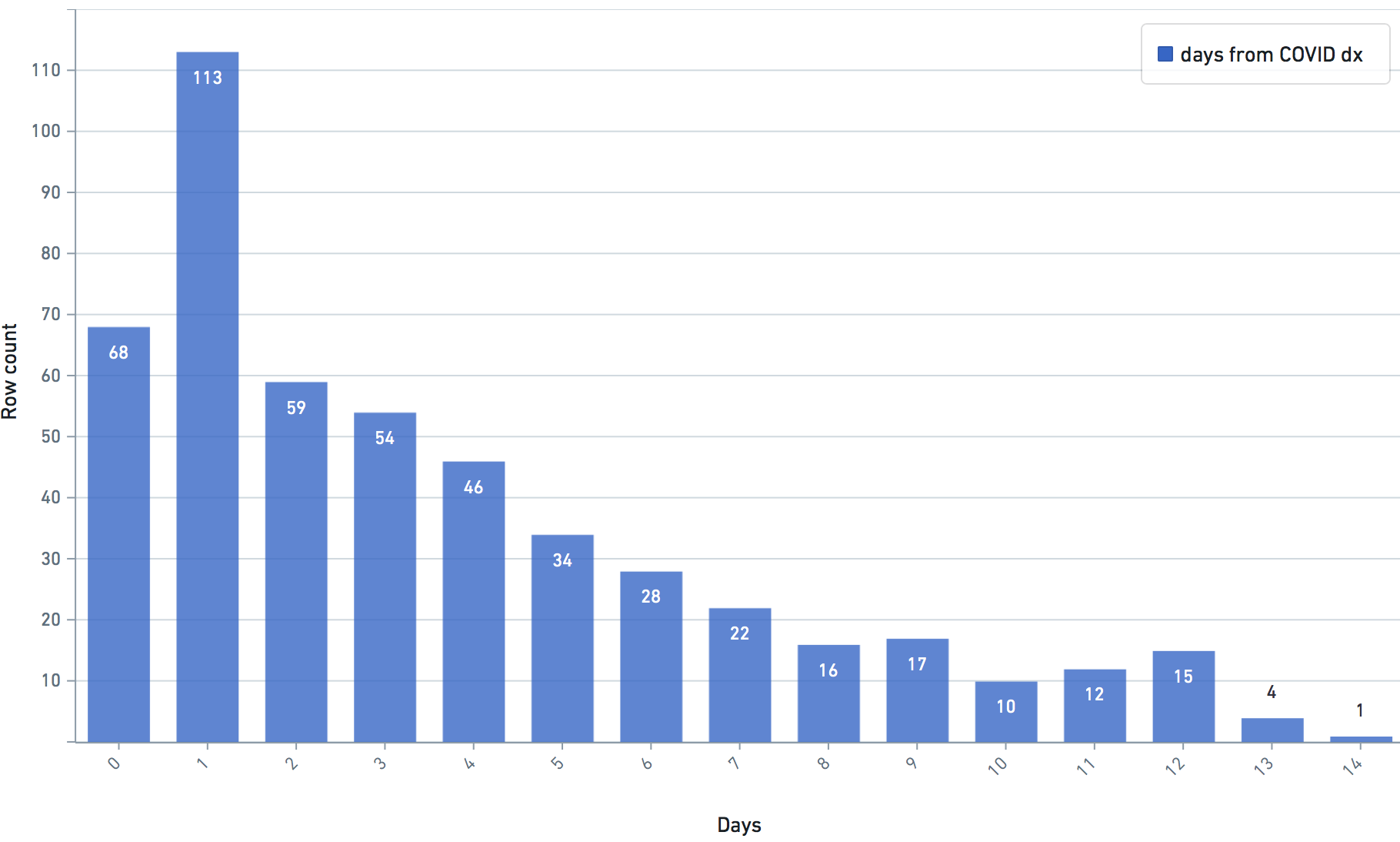
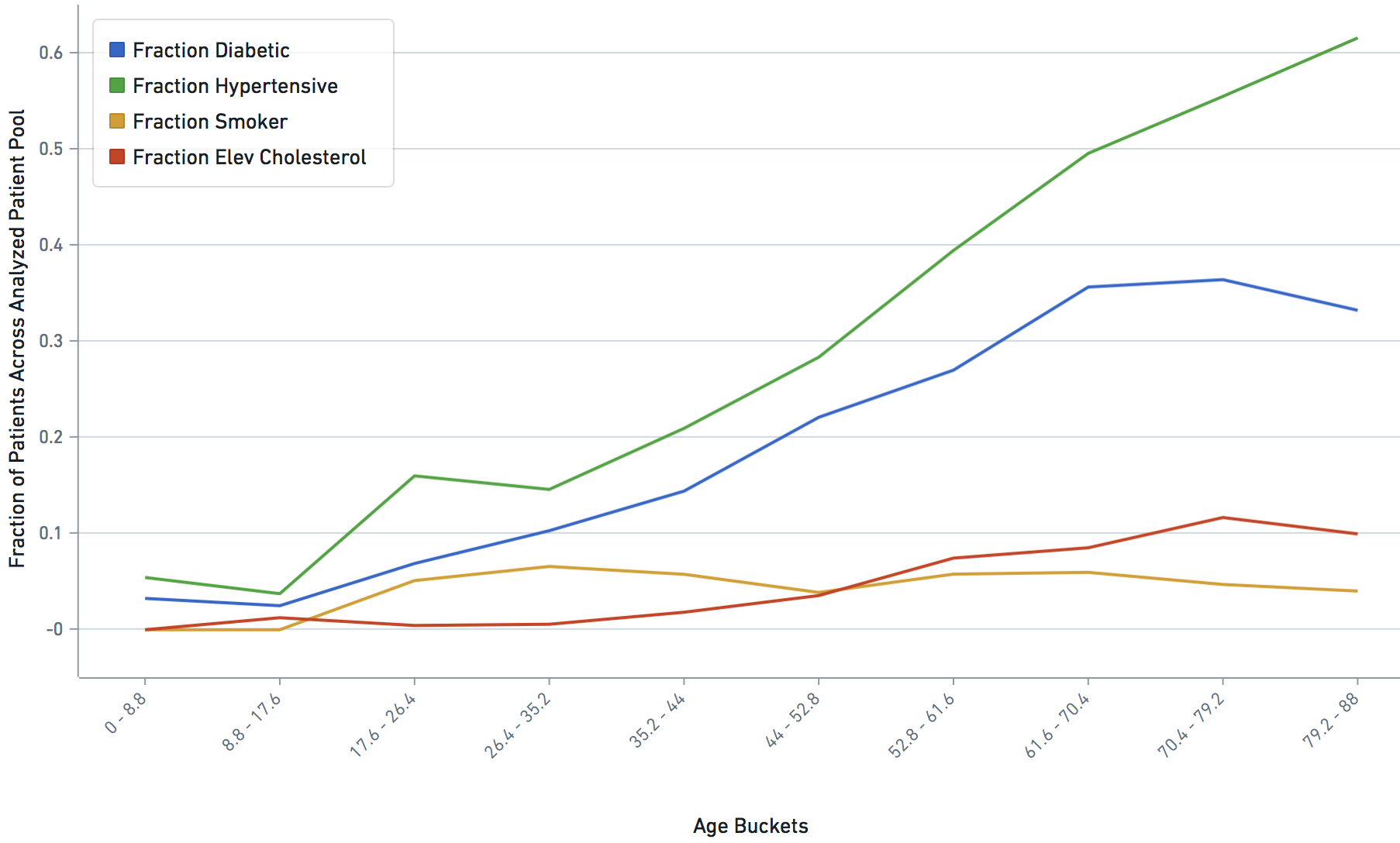
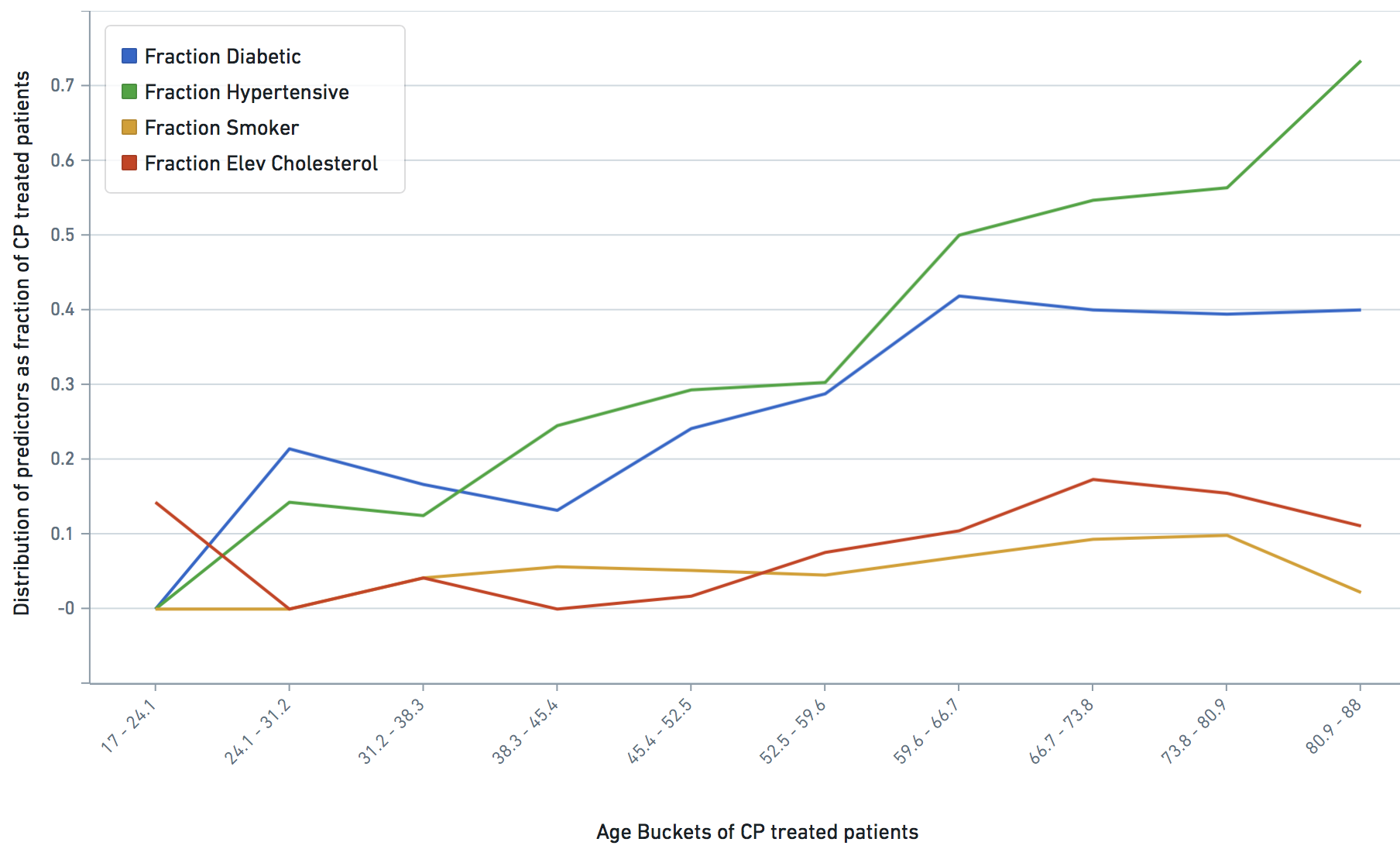
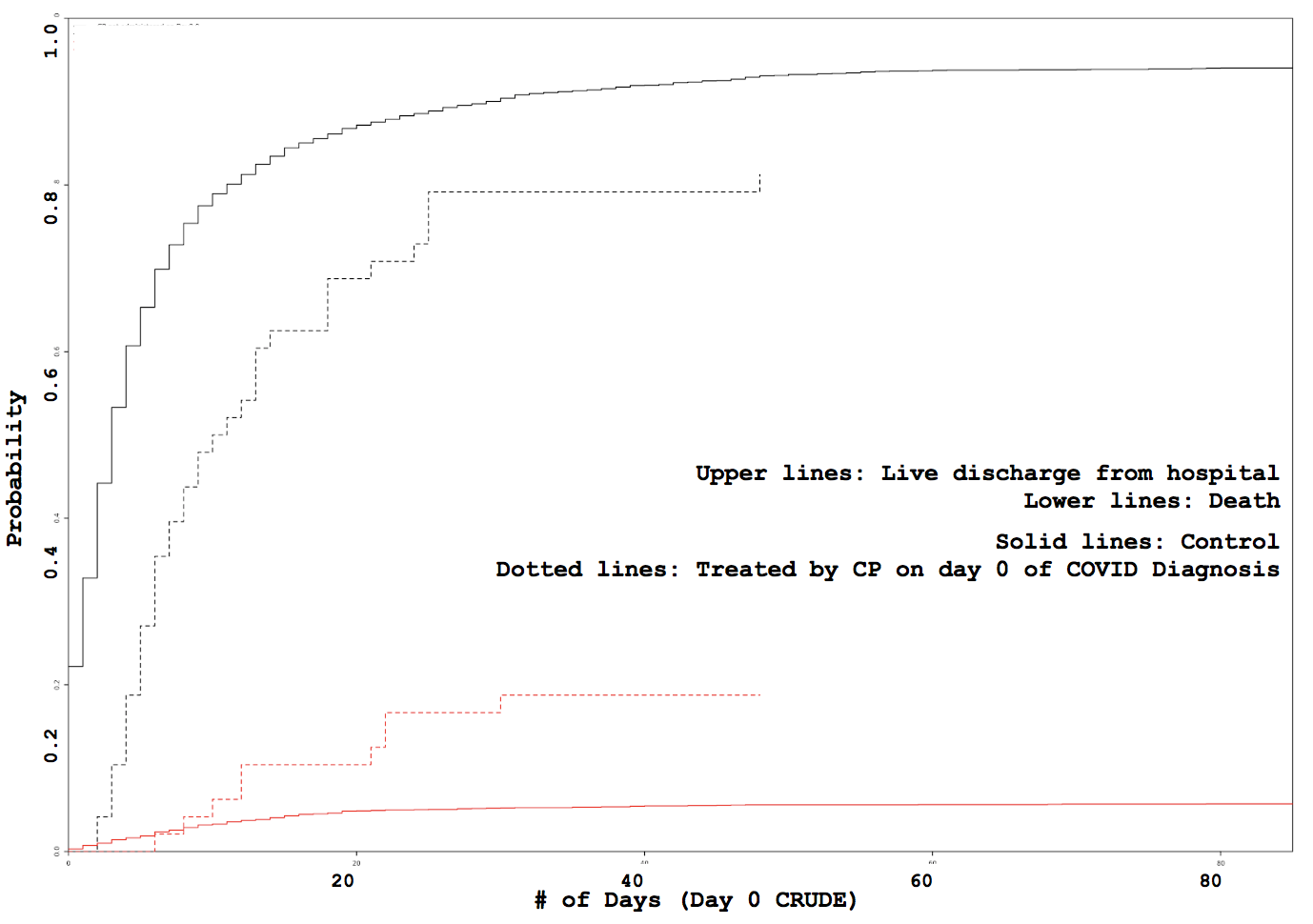
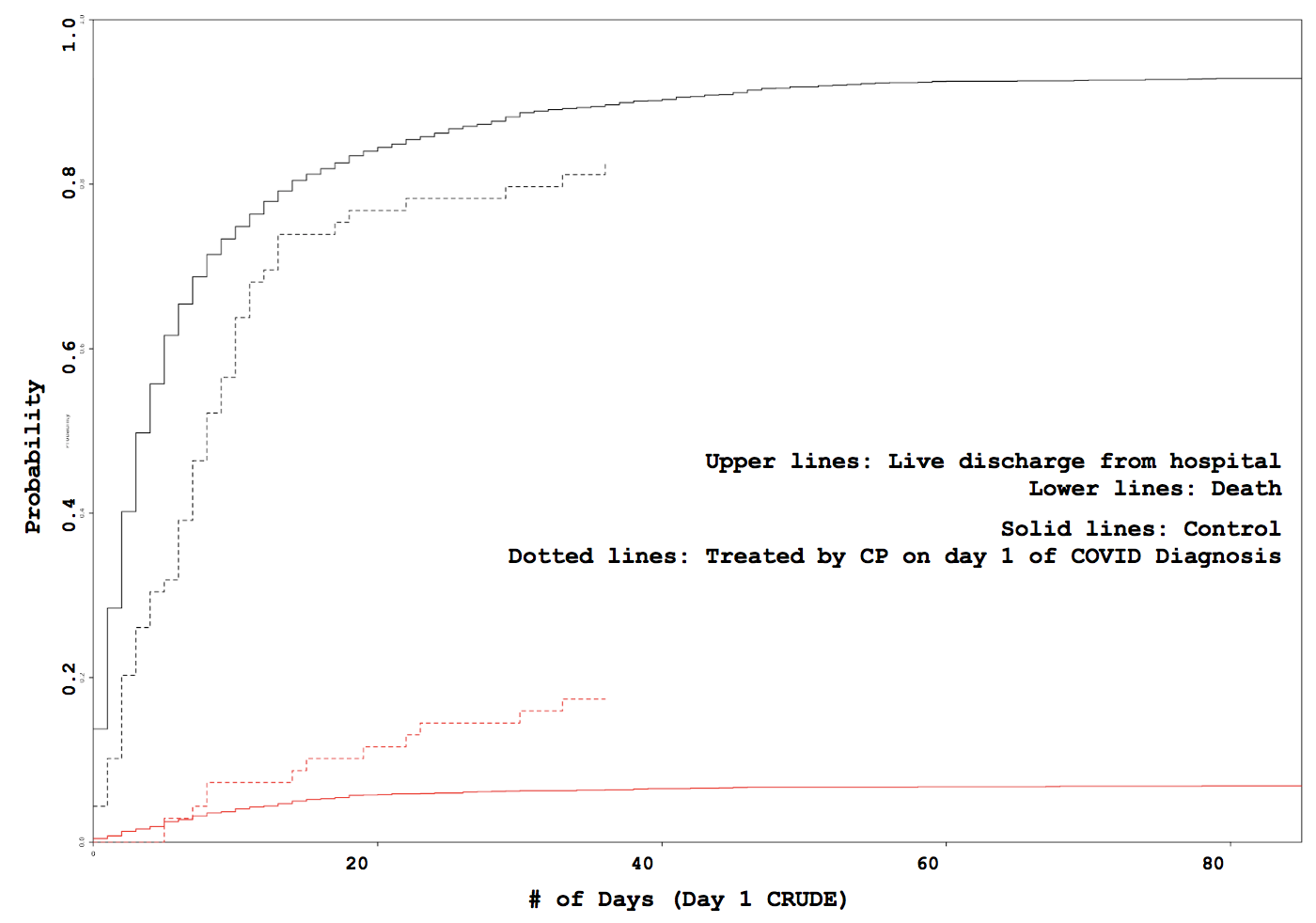
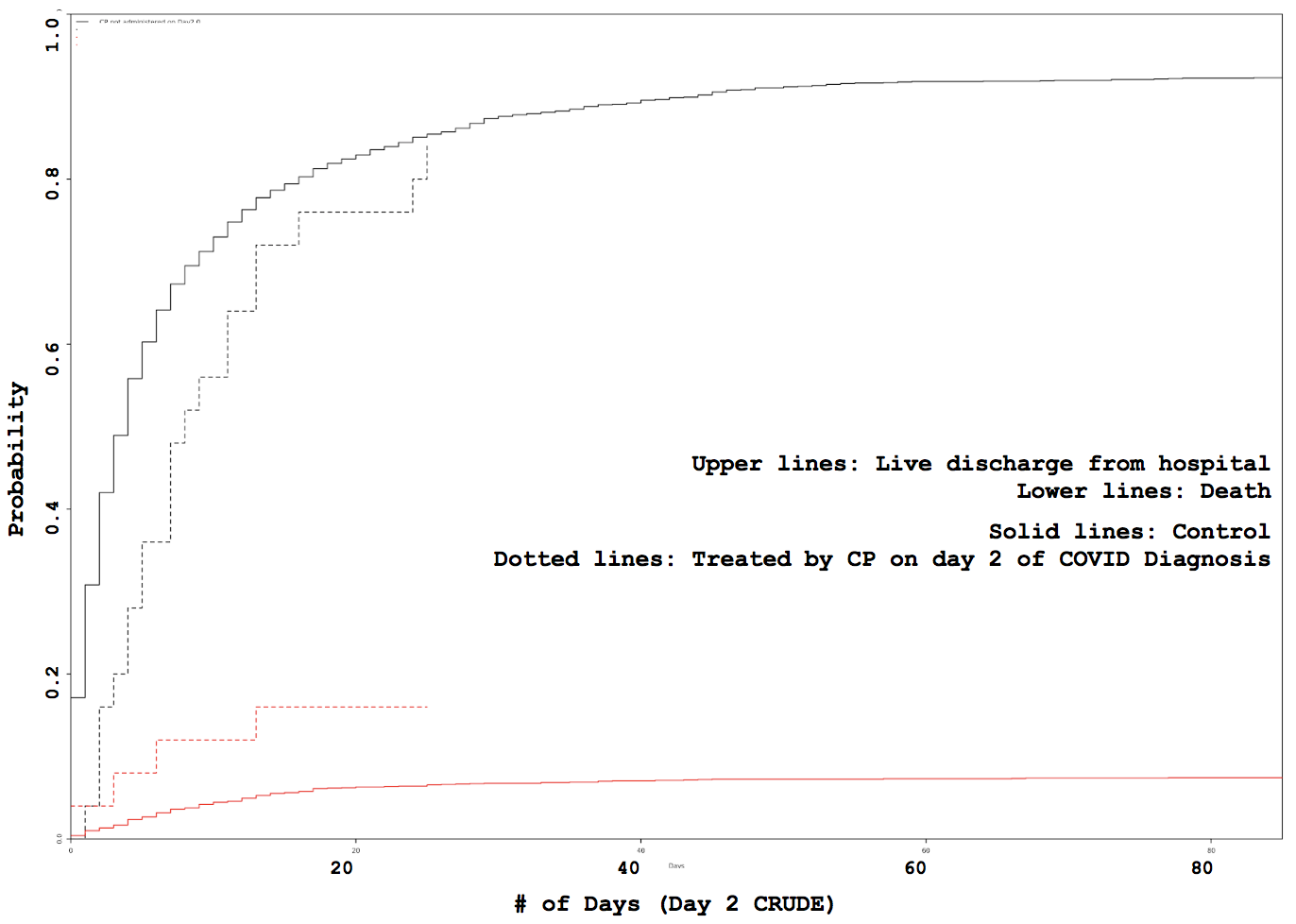
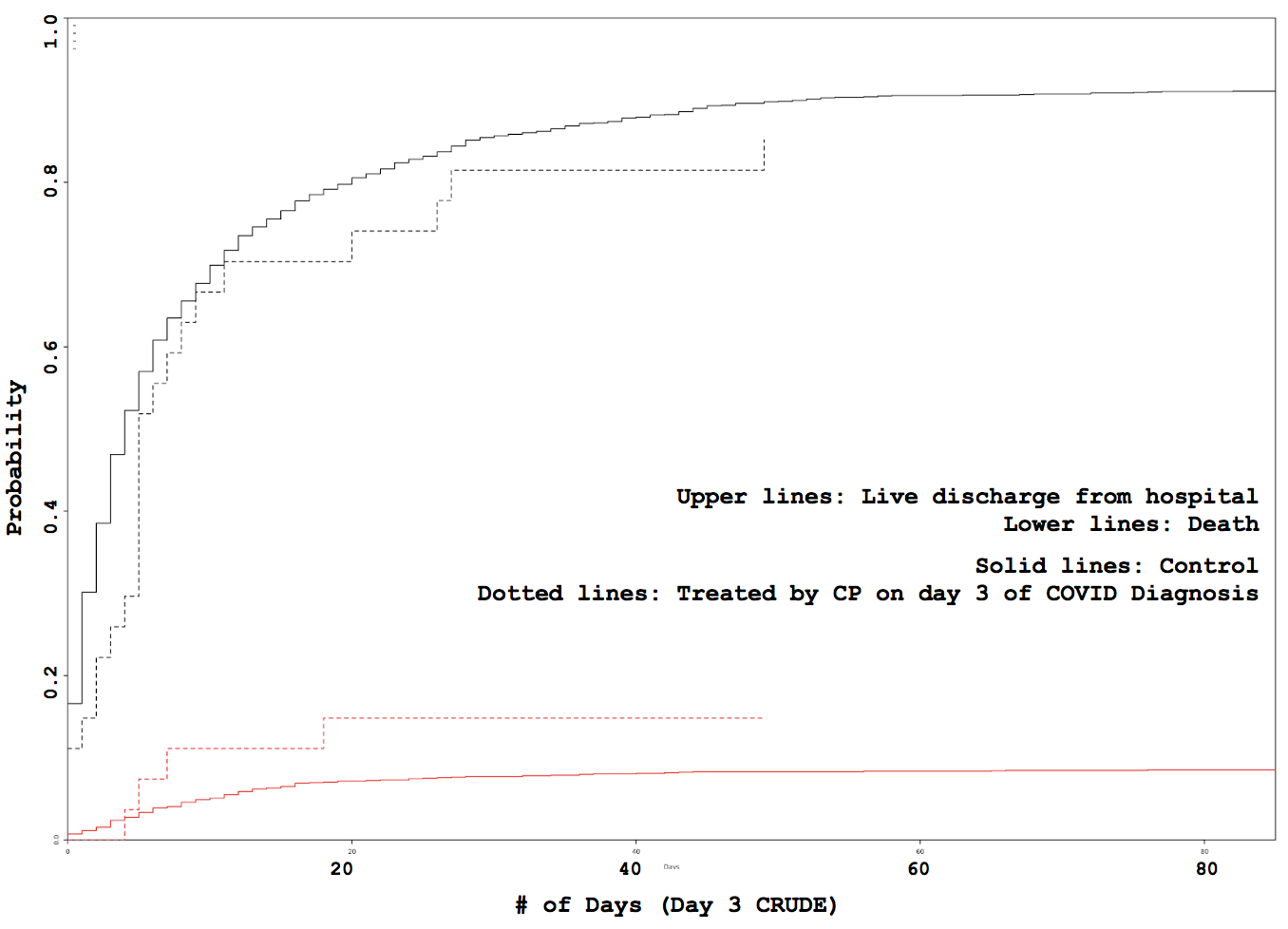
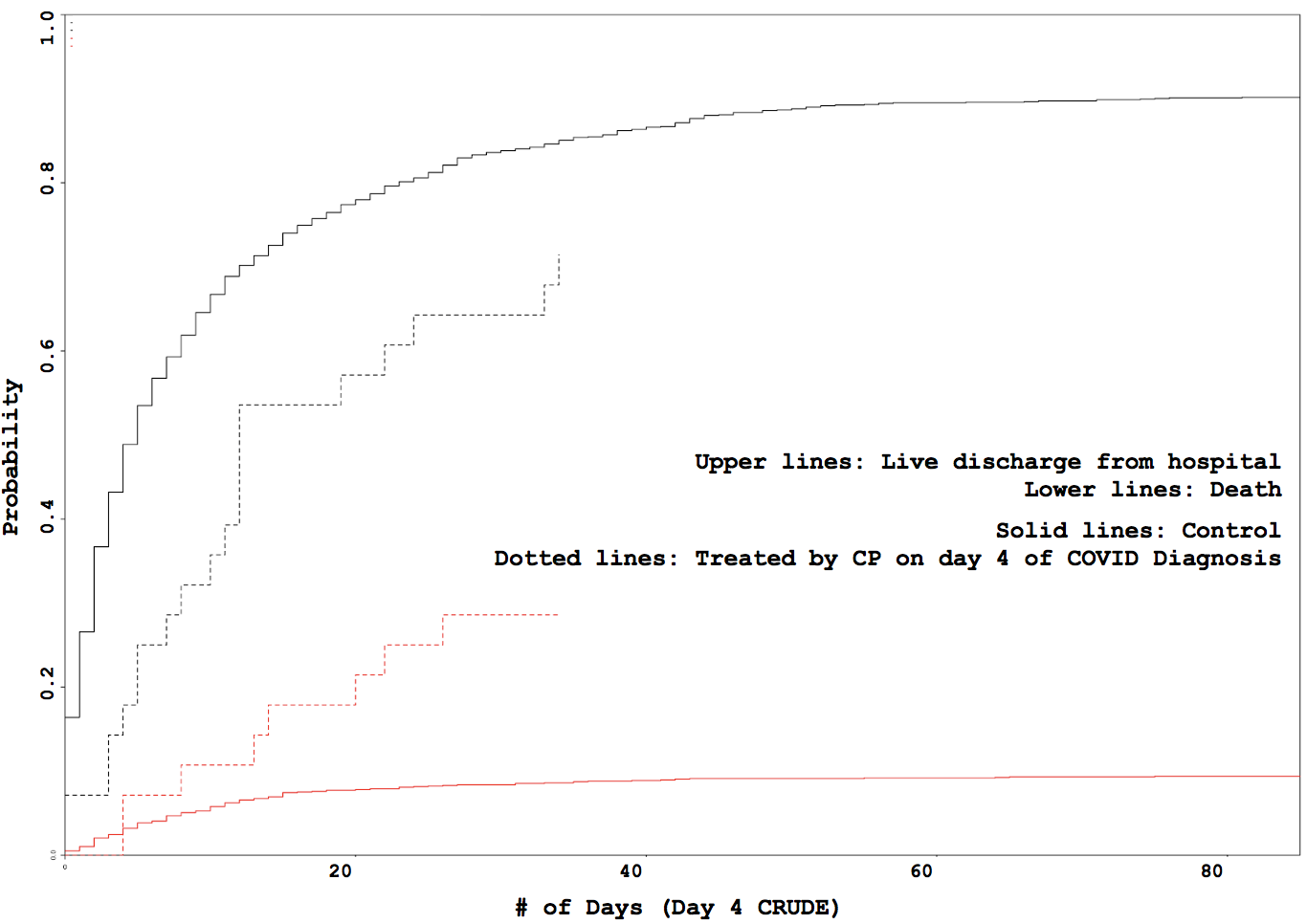
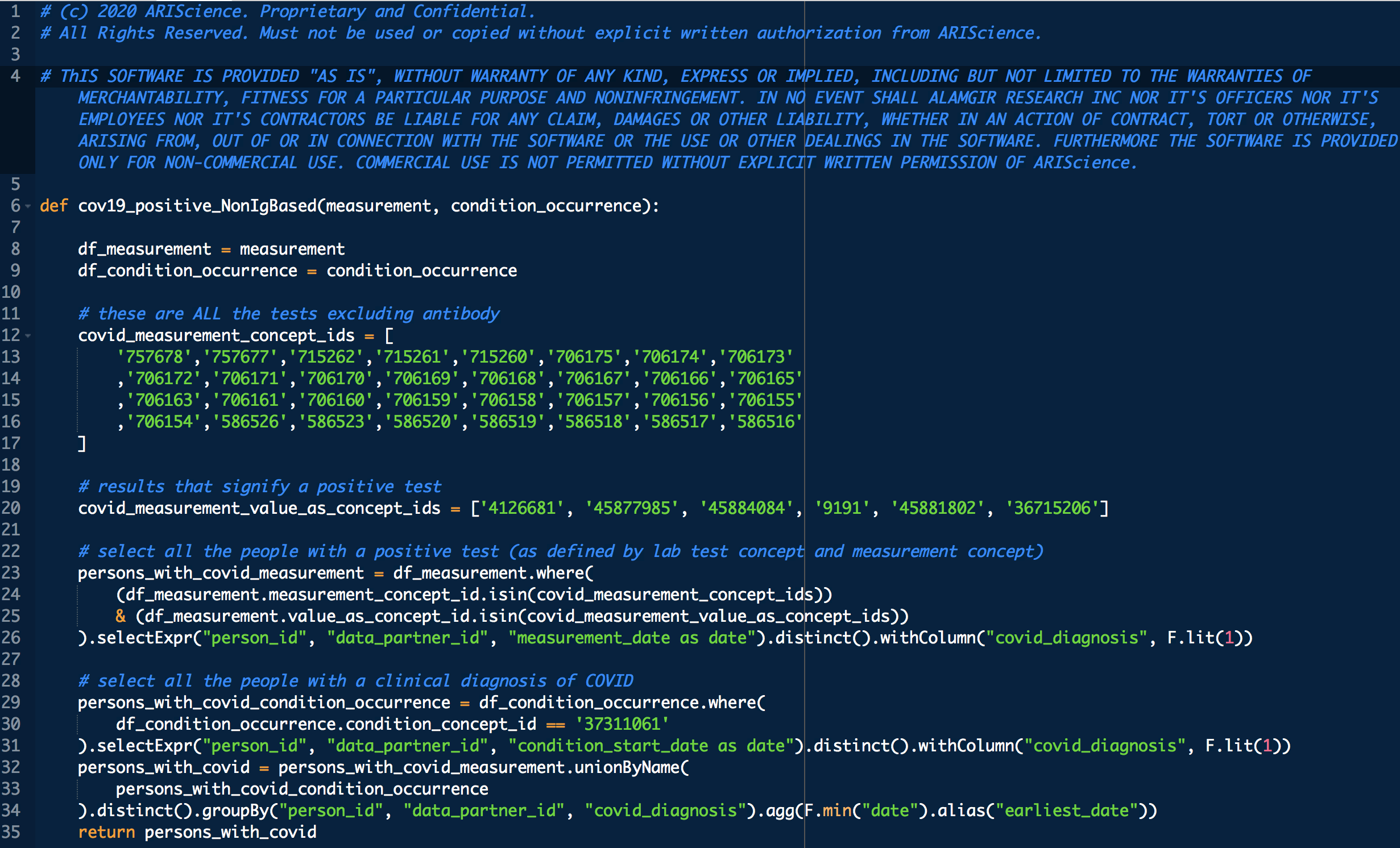
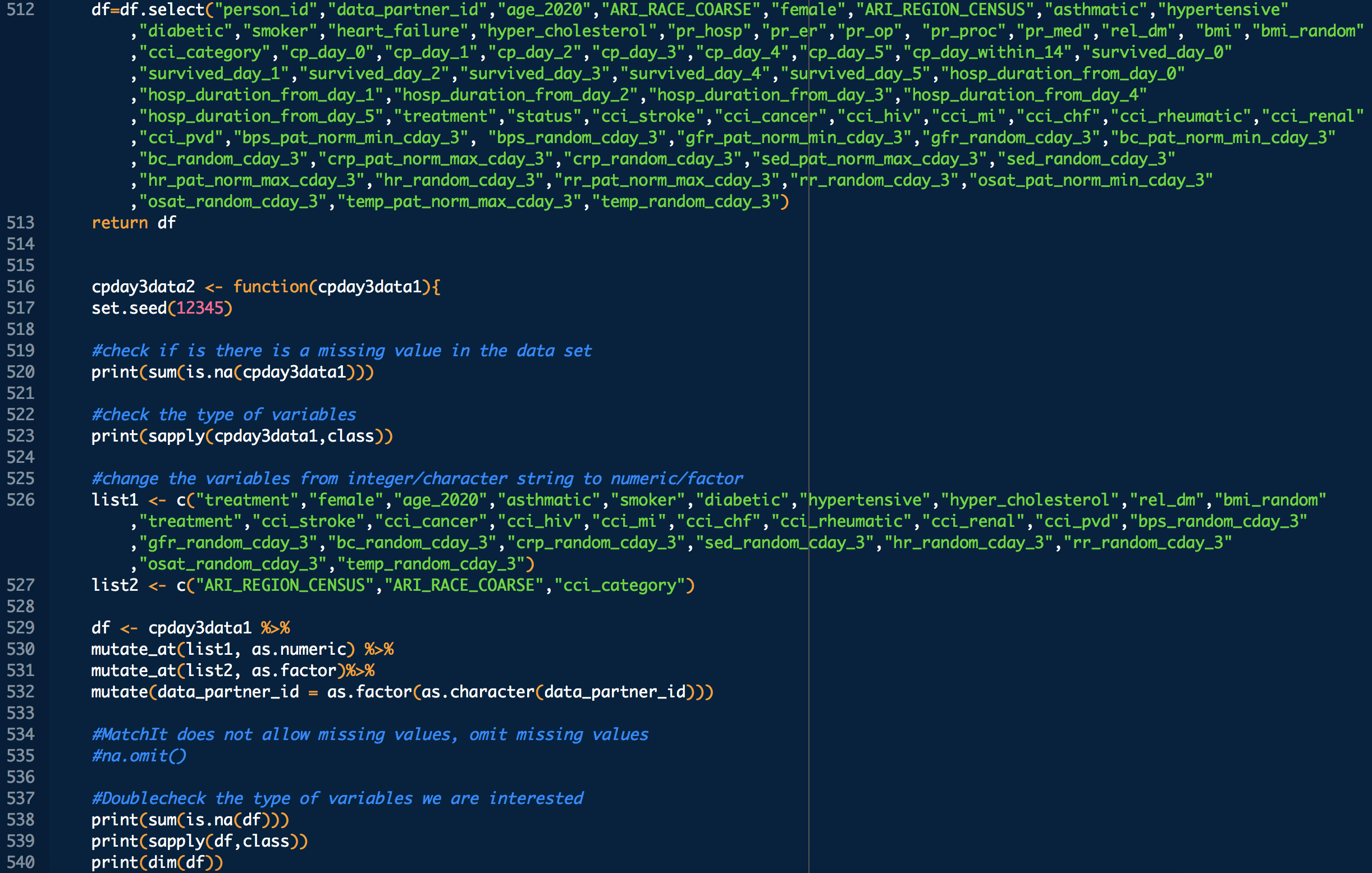
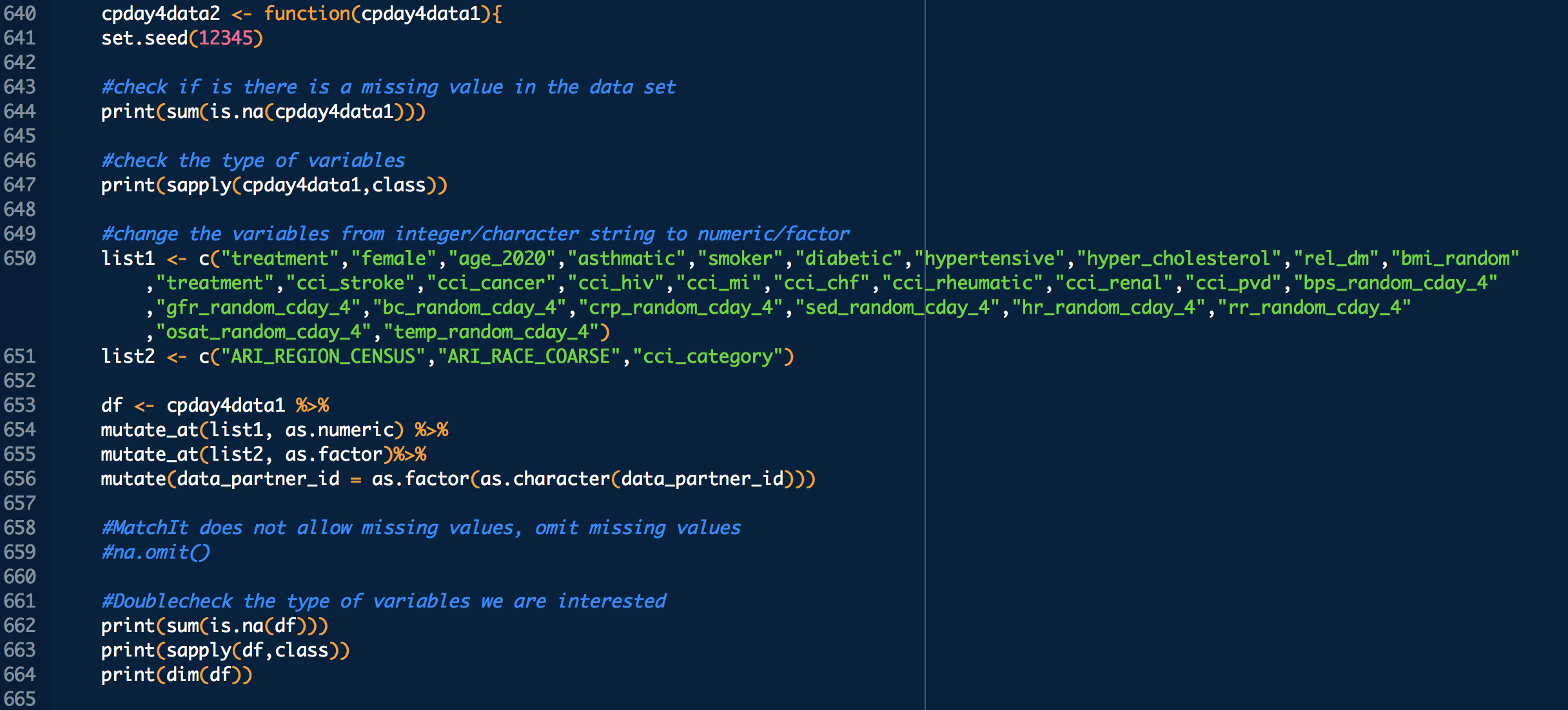
Some VARIABLE definitions:
PRE COVID-19:
female = 1 means female, otherwise male
age_2020 = age in the year 2020
ARI_REGION_CENSUS = census regions
ARI_RACE_COARSE = race
asthmatic = 1 prior chronic respiratory disorder, 0 otherwise
smoker = 1,0 self explanatory
diabetic = 1 prior diabetes diagnosis, 0 otherwise
hypertensive = 1 prior hypertension diagnosis, 0 otherwise
cci_category = 0->A, 1->B , 2 and 3 -> C, 4 -> D, 5+ -> E
rel_dm = covid related dexamethasone use
hyper_cholesterol = 0 -> no evidence of elevated cholesterol, 1->evidence of elevated cholesterol
cci_stroke = 1 if there was evidence of stroke, an underlying calculation factor for Charlson Comorbidity Index, 0 otherwise
cci_cancer = 1 if there was evidence of stroke, an underlying calculation factor for Charlson Comorbidity Index, 0 otherwise
cci_hiv = 1 if there was evidence of stroke, an underlying calculation factor for Charlson Comorbidity Index, 0 otherwise
cci_mi = 1 if there was evidence of stroke, an underlying calculation factor for Charlson Comorbidity Index, 0 otherwise
cci_chf = 1 if there was evidence of stroke, an underlying calculation factor for Charlson Comorbidity Index, 0 otherwise
cci_rheumatic = 1 if there was evidence of stroke, an underlying calculation factor for Charlson Comorbidity Index, 0 otherwise
cci_renal = 1 if there was evidence of stroke, an underlying calculation factor for Charlson Comorbidity Index, 0 otherwise
cci_pvd = 1 if there was evidence of stroke, an underlying calculation factor for Charlson Comorbidity Index, 0 otherwise
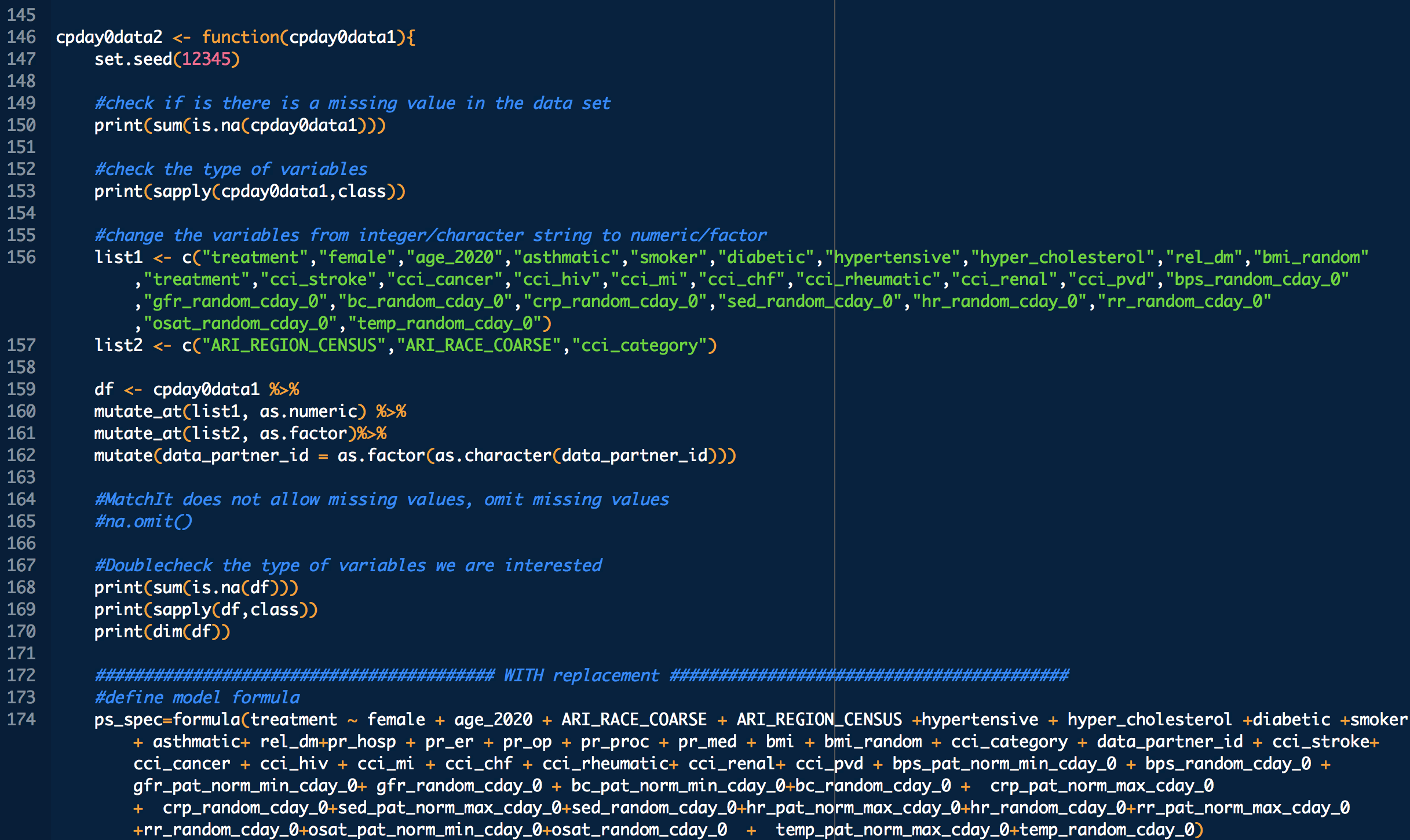
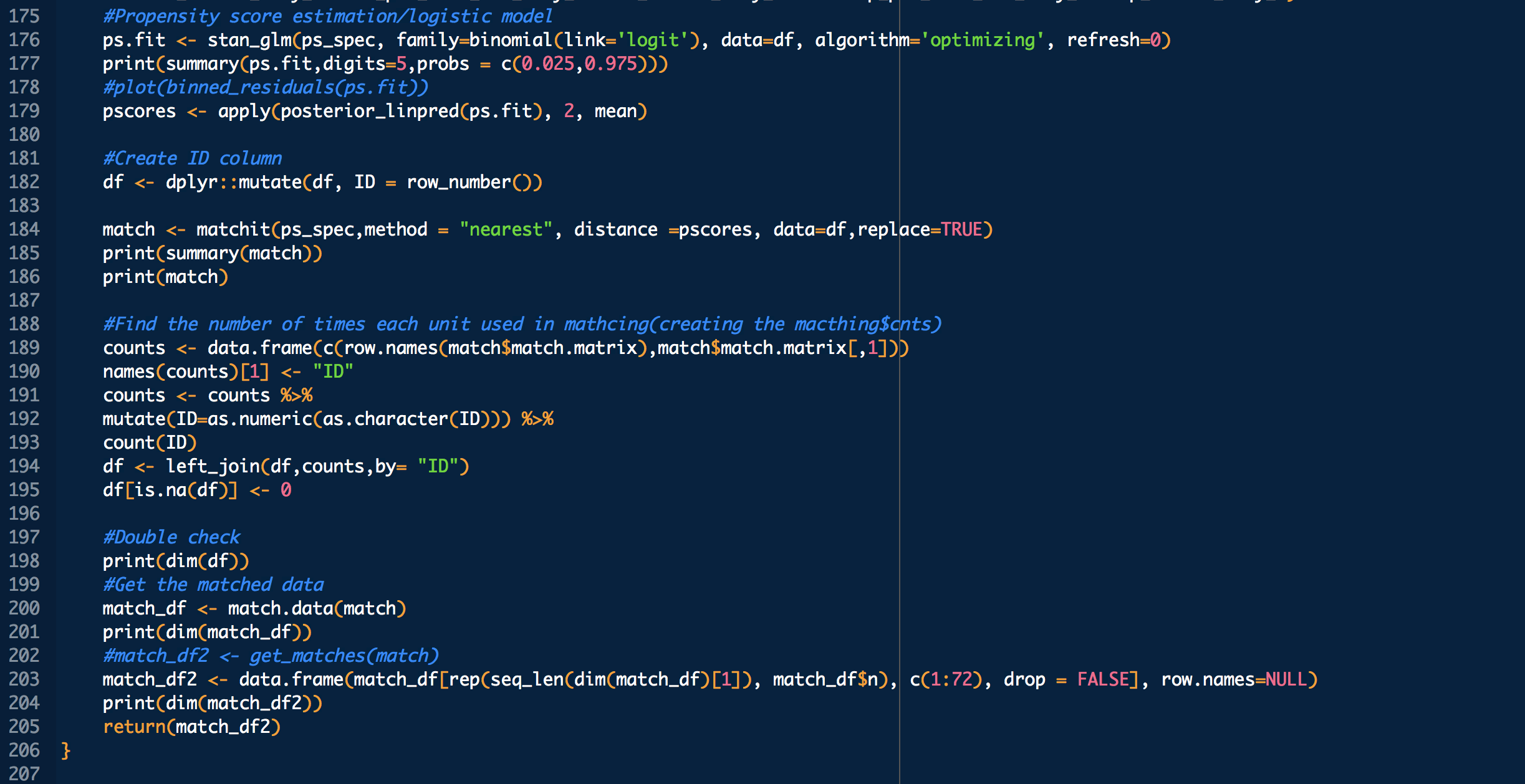
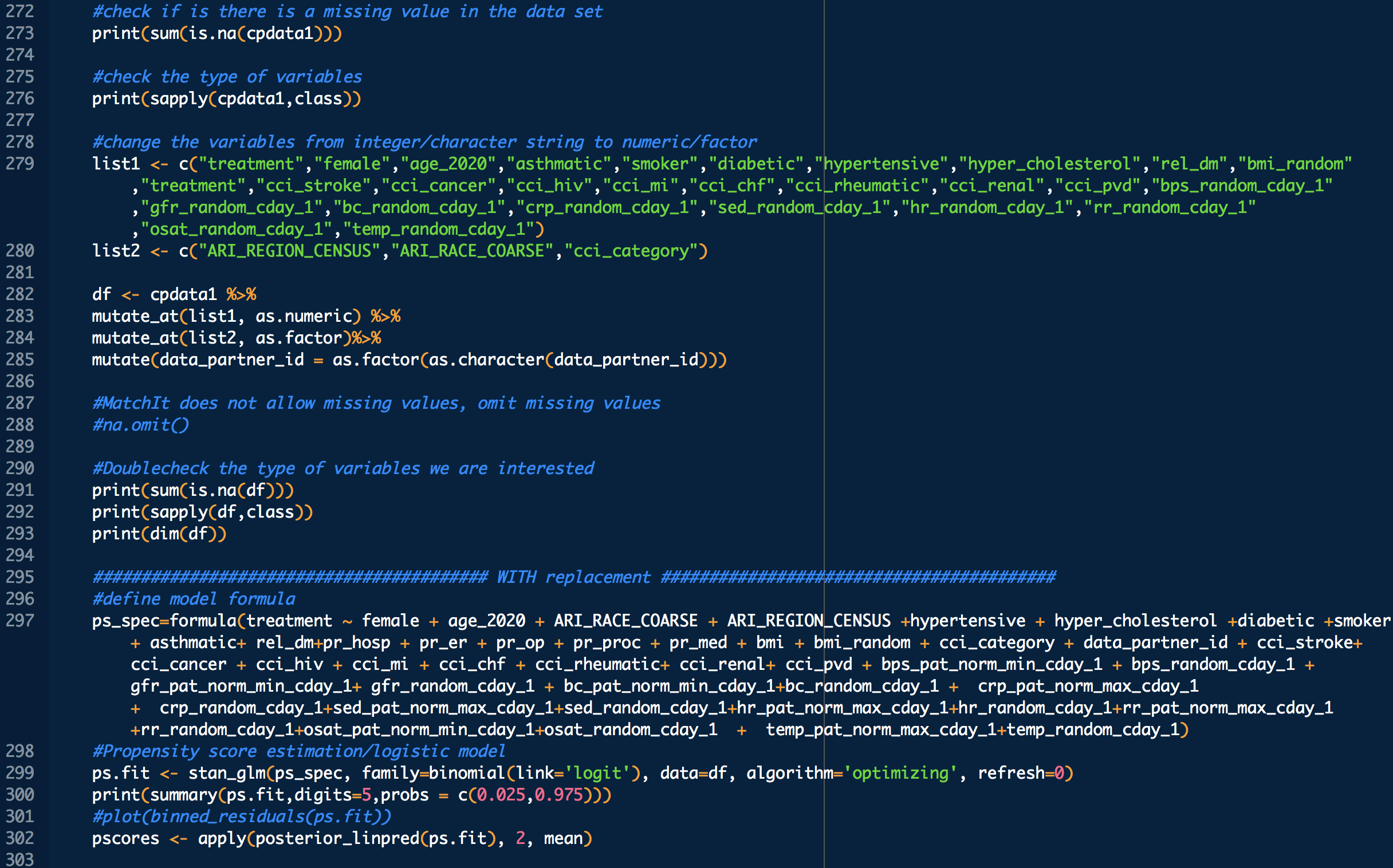
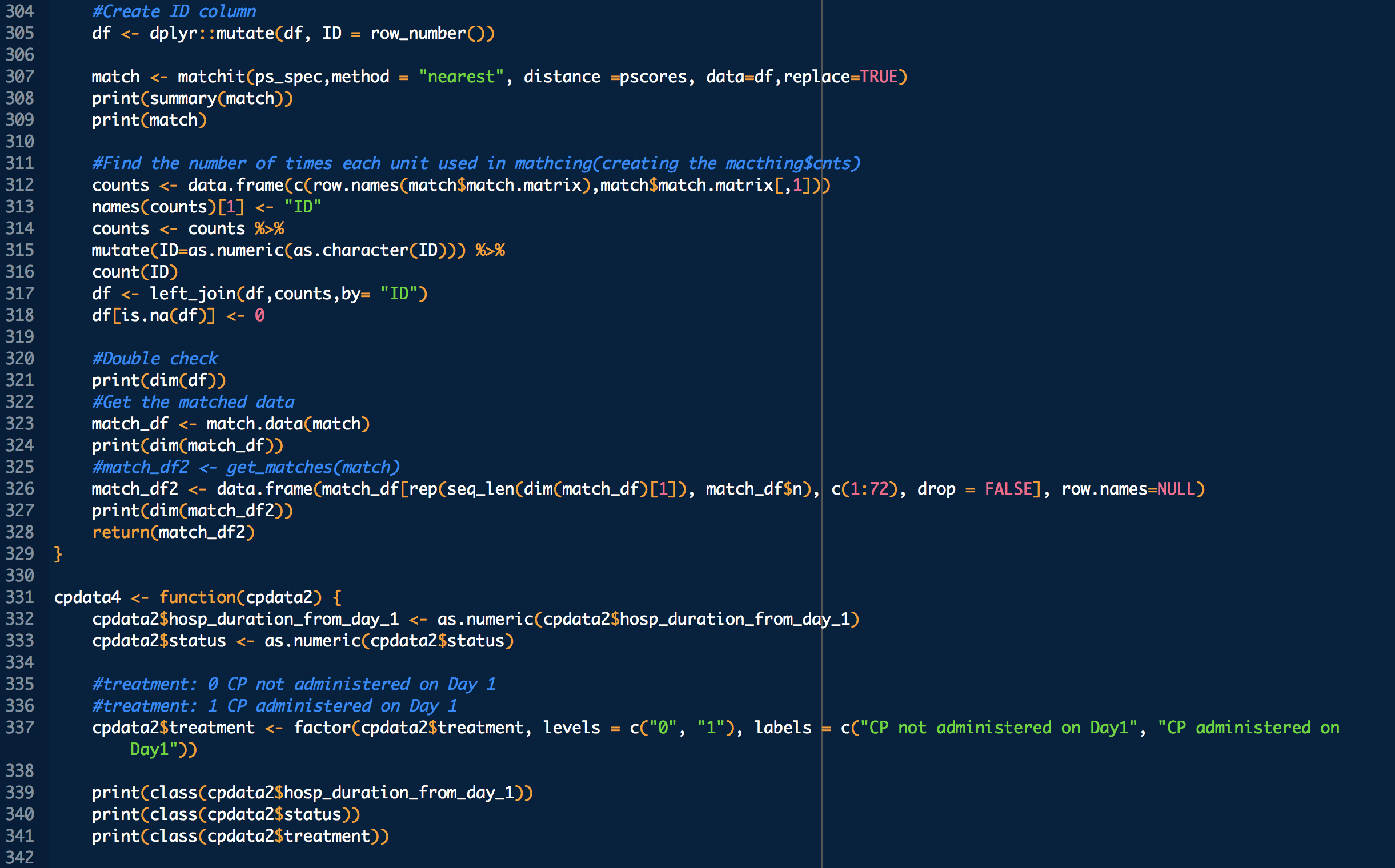
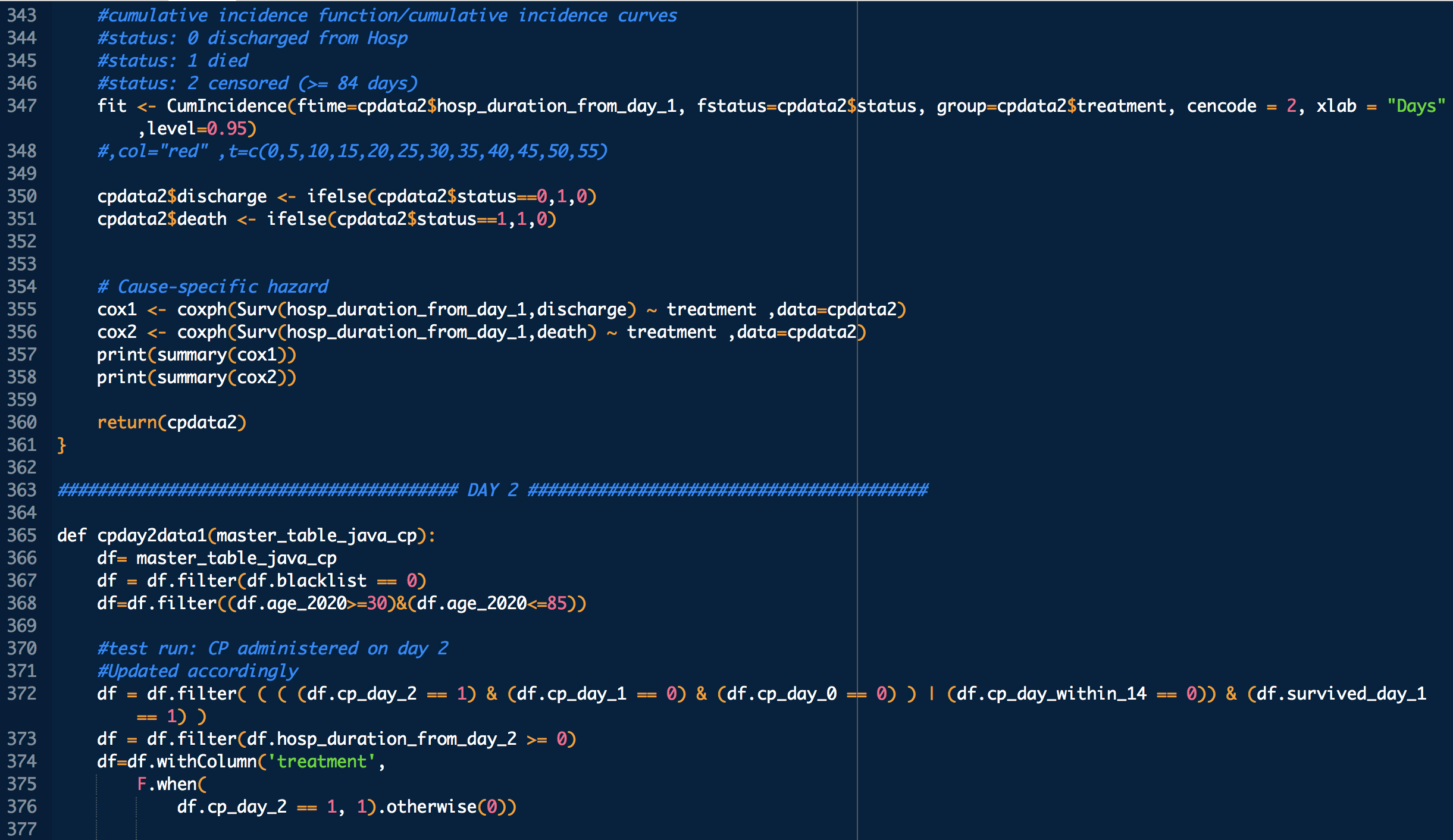
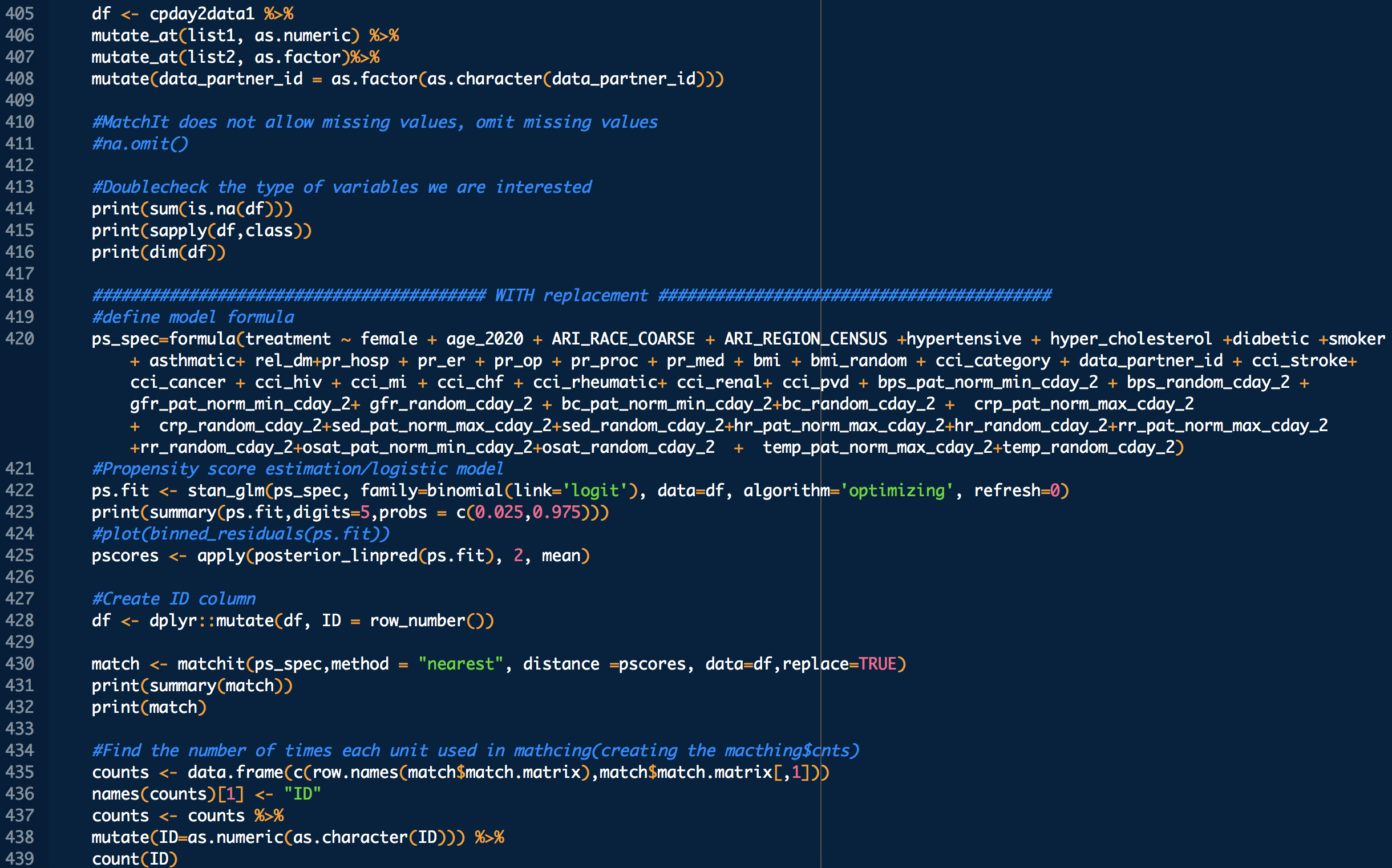
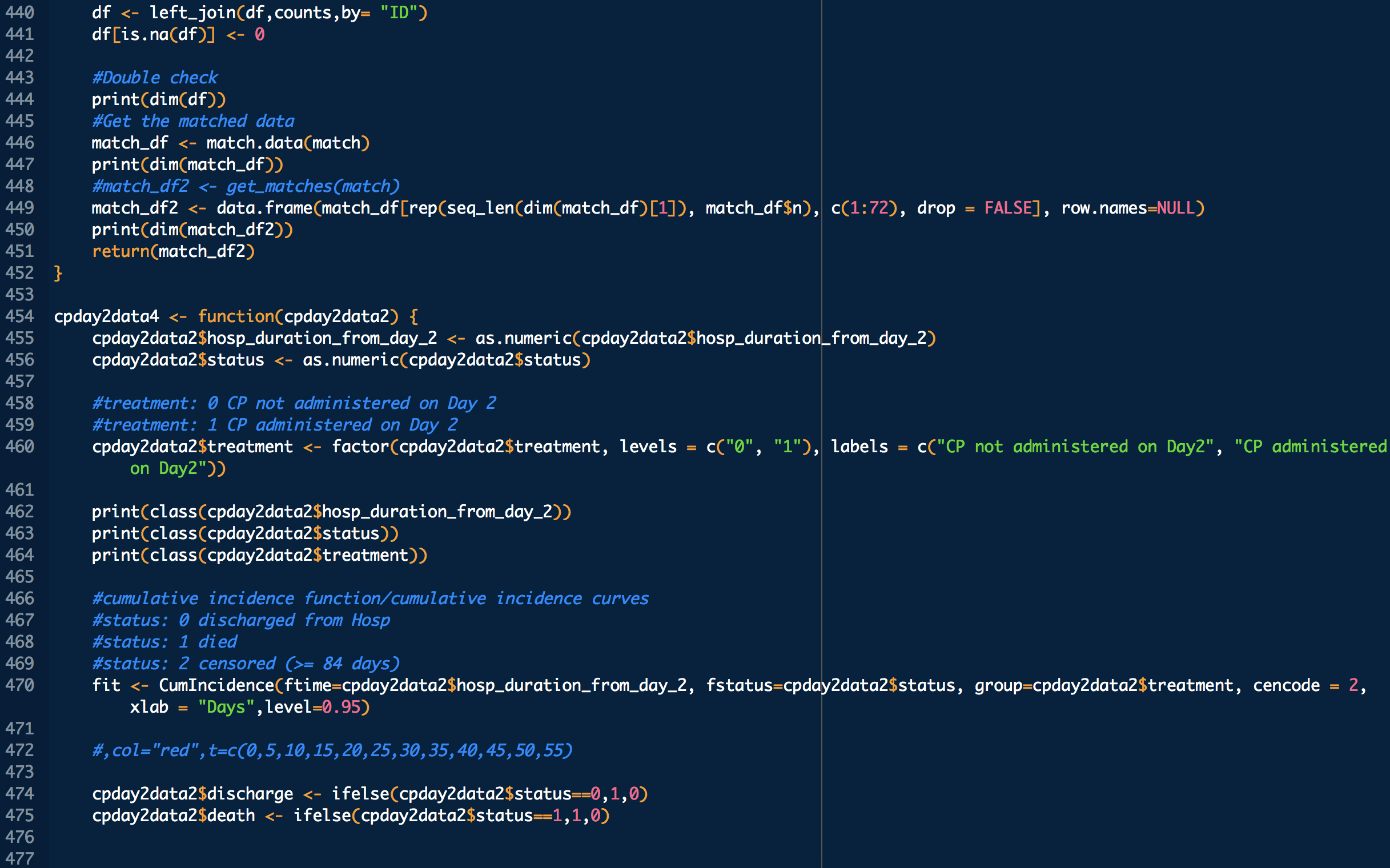
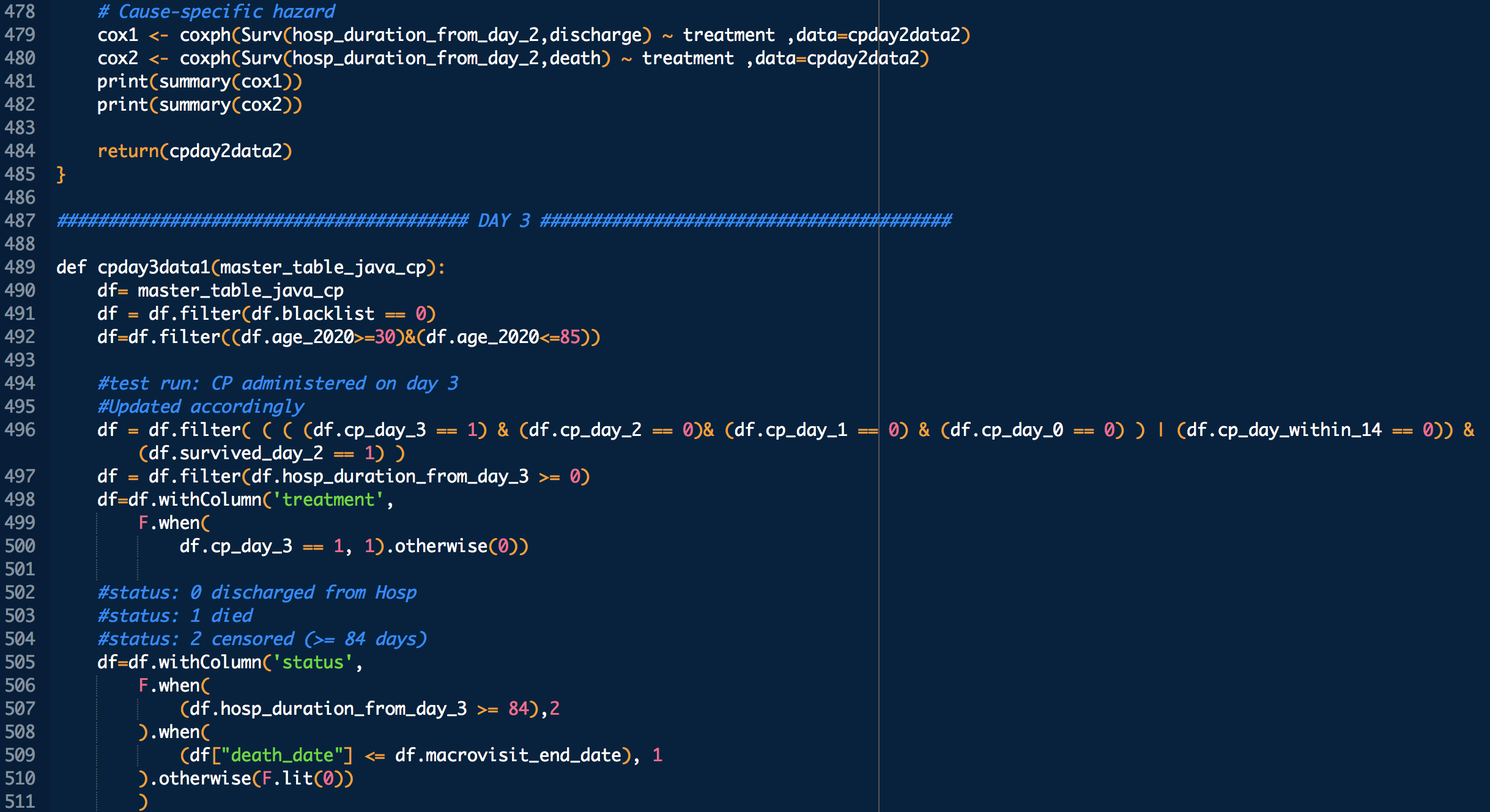
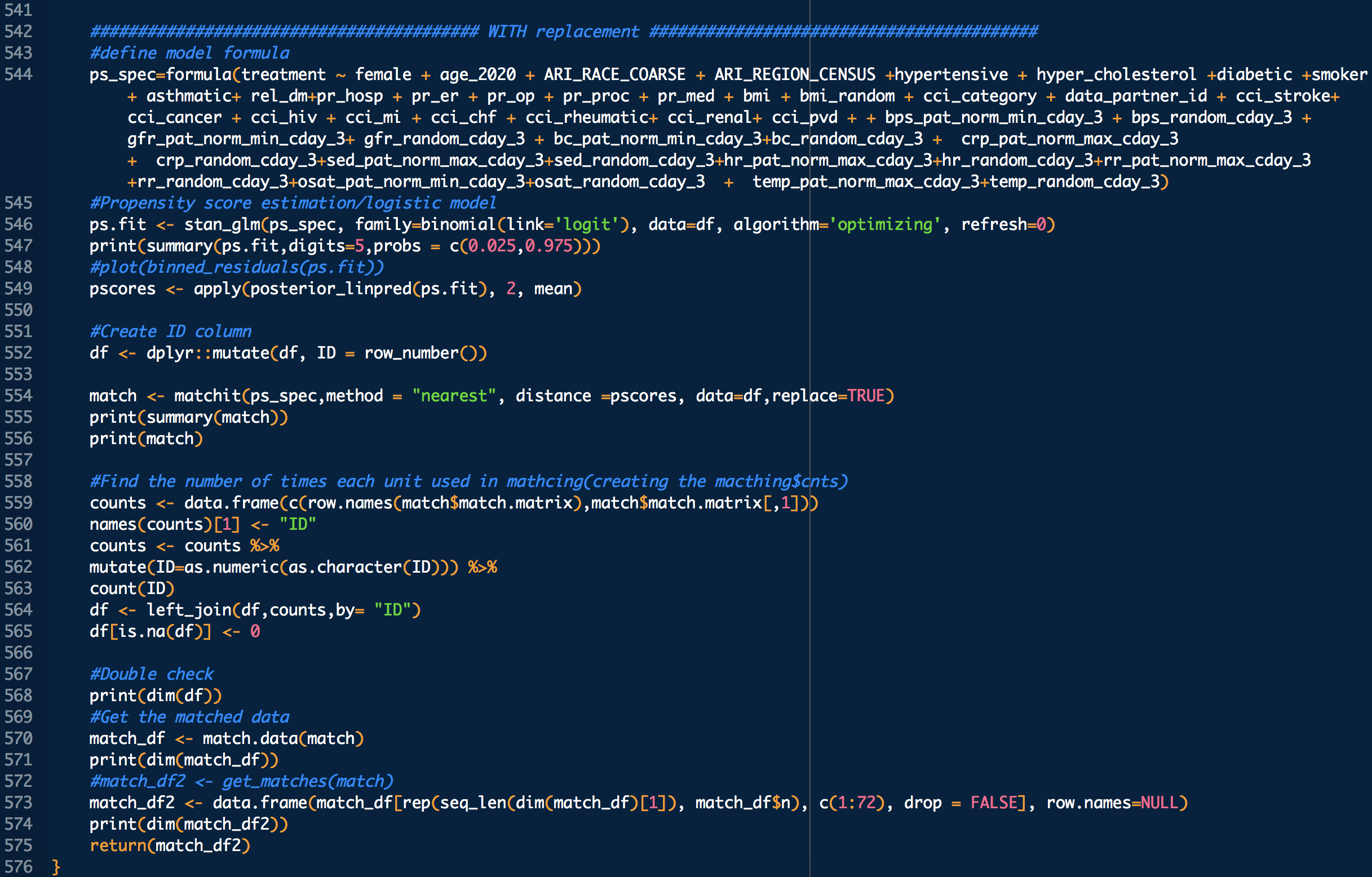
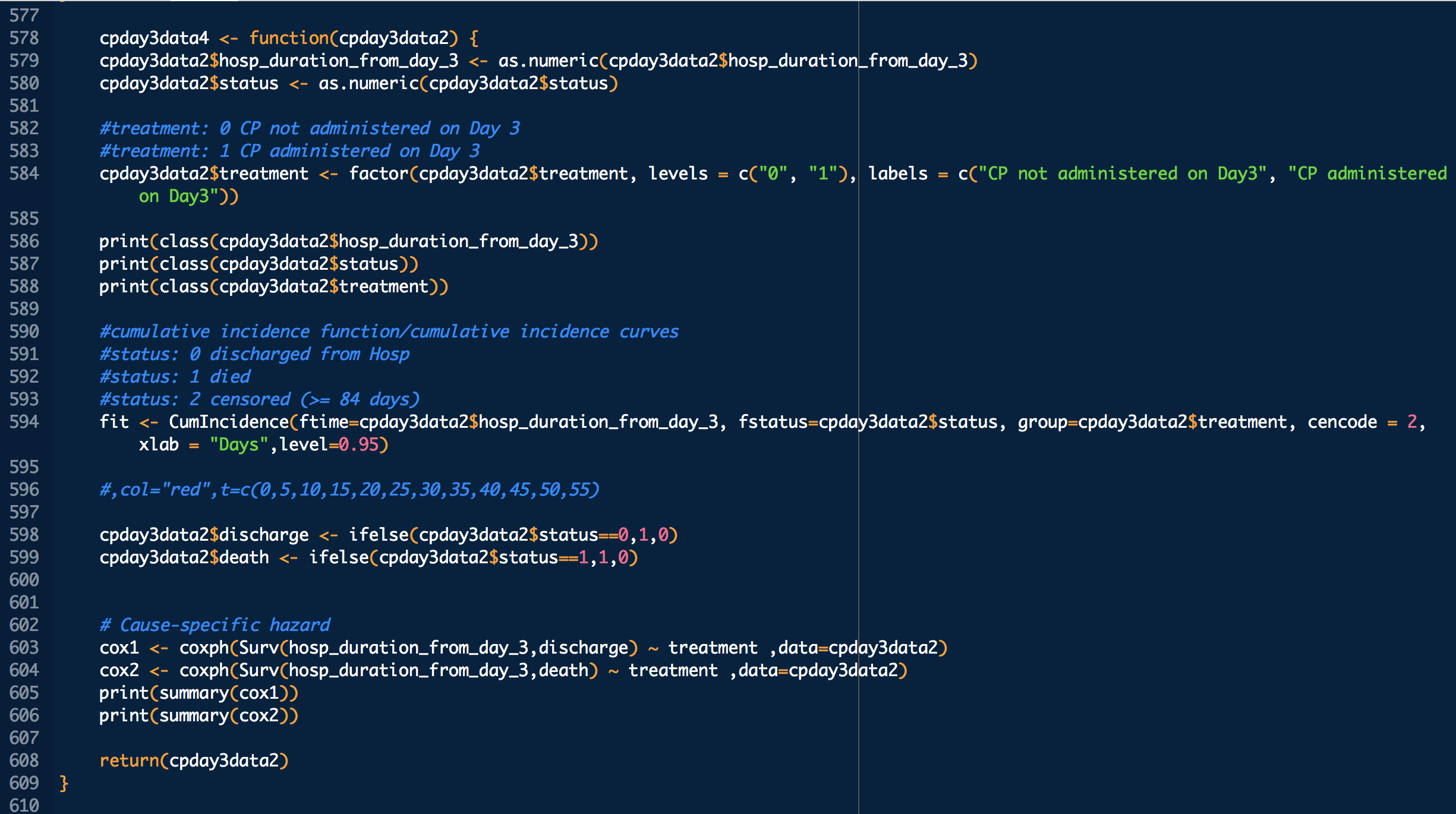
treatment: 1 means treated (with CP), 0 means untreated
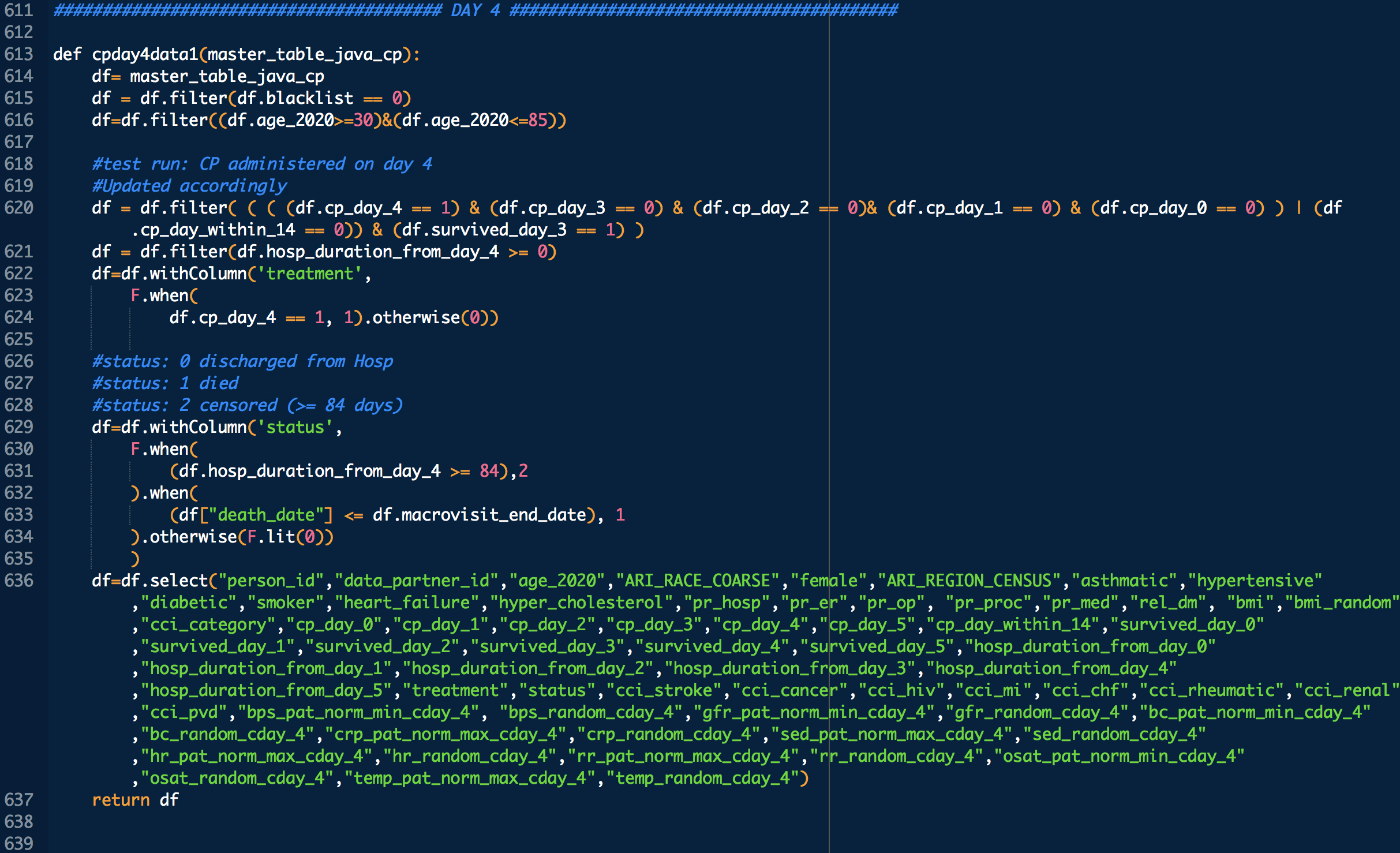
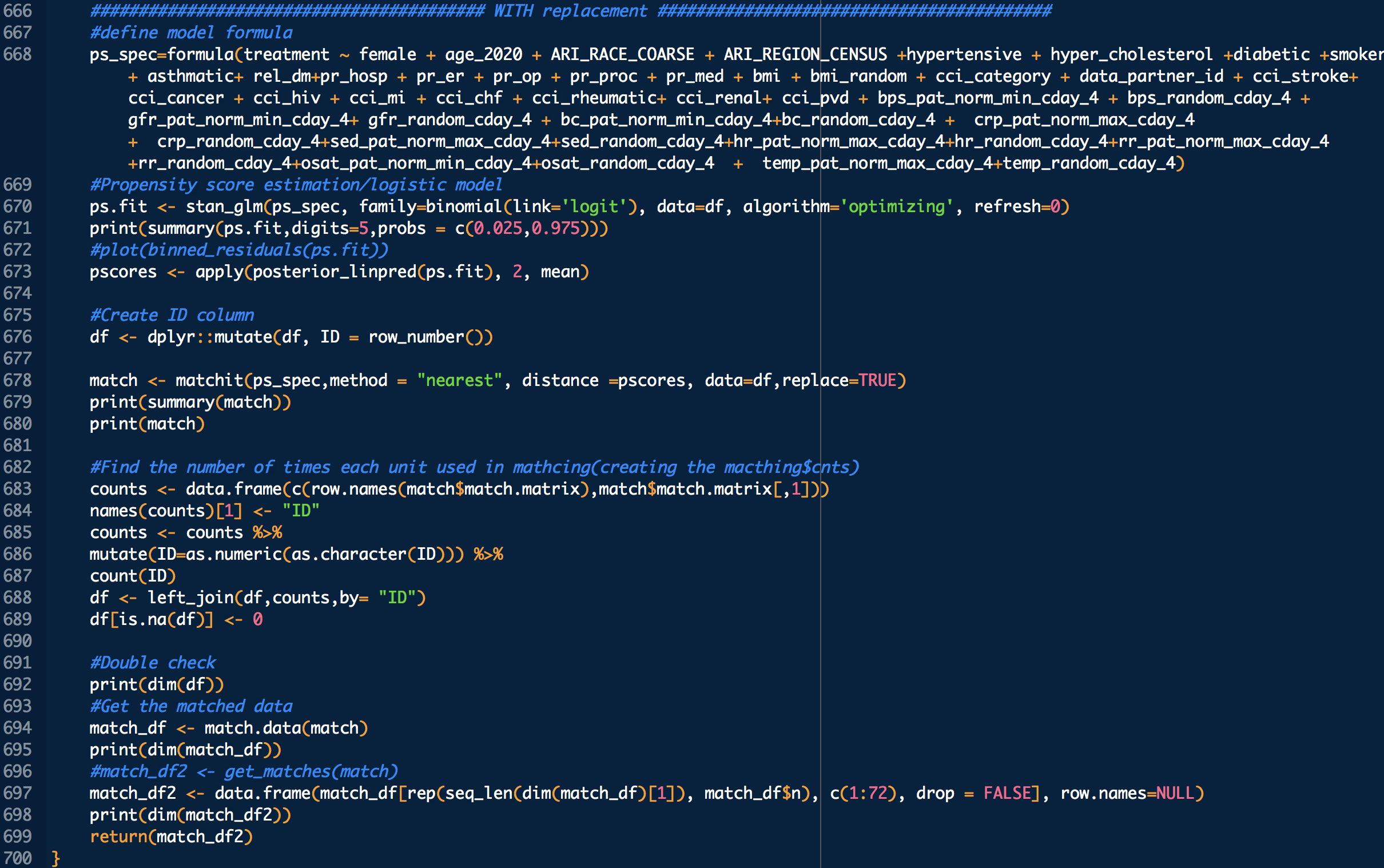
DURING COVID-19 (N below means day of COVID-19 diagnosis, N = 0,1,2,3,4 in this study)
bps_pat_norm_min_cday_N = minimum systolic blood pressure of patient on day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
bps_random_cday_N = 1 means there was missing data for blood pressure, 0 means there actual data
gfr_pat_norm_min_cday_N = minimum GFR on day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
gfr_random_cday_N = 1 means there was missing data for gfr, 0 means there actual data
bc_pat_norm_min_cday_N = minimum bi-carbonate on day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
bc_random_cday_N = 1 means there was missing data, 0 means there actual data
crp_pat_norm_max_cday_N = maximum C reactive protein on day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
crp_random_cday_N = 1 means there was missing data for C reactive protein, 0 means there actual data
sed_pat_norm_max_cday_N = maximum SED rate on day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
sed_random_cday_N = 1 means there was missing data for SED rate, 0 means there actual data
hr_pat_norm_max_cday_N = maximum heart rate on day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
hr_random_cday_N = 1 means there was missing data for heart rate, 0 means there actual data
rr_pat_norm_max_cday_N = maximum respiratory rate on day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
rr_random_cday_N = 1 means there was missing data for respiratory, 0 means there actual data
osat_pat_norm_min_cday_N = maximum O2 saturation day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
osat_random_cday_N = 1 means there was missing data for O2 saturation, 0 means there actual data
temp_pat_norm_max_cday_N = maximum body temperature on day N of COVID-19 diagnosis, range normalized betweeen 0 and 1
temp_random_cday_N = 1 means there was missing data for body temperature, 0 means there actual data

By using any of these resources ("SOFTWARE") in this site you agree that:
THE SOFTWARE IS PROVIDED "AS IS", WITHOUT WARRANTY OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO THE WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NONINFRINGEMENT. IN NO EVENT SHALL ALAMGIR RESEARCH INC NOR IT'S OFFICERS NOR IT'S EMPLOYEES NOR IT'S CONTRACTORS BE LIABLE FOR ANY CLAIM, DAMAGES OR OTHER LIABILITY, WHETHER IN AN ACTION OF CONTRACT, TORT OR OTHERWISE, ARISING FROM, OUT OF OR IN CONNECTION WITH THE SOFTWARE OR THE USE OR OTHER DEALINGS IN THE SOFTWARE. FURTHERMORE THE SOFTWARE IS PROVIDED ONLY FOR NON-COMMERCIAL USE. COMMERCIAL USE IS NOT PERMITTED WITHOUT EXPLICIT WRITTEN PERMISSION OF ARIScience.